Introduction
The chest wall can display a variety of abnormalities. These range from pectus carinatum (PC), the second most common deformity, characterized by a protruding chest [1], to pectus excavatum (PE) or “funnel chest”, which is the most prevalent anterior chest wall deformity (CWD), where the chest appears sunken in [2, 3]. In certain instances, such as Poland syndrome, there may be a total absence of ribs and pectoral muscles [2]. PE can be readily corrected using minimally invasive methods, and PC can be rectified using safe, established traditional techniques [4]. Approximately 1% of the population is affected by pectus deformities (PDs) [4].
Indexes of cardiac deformity, like the cardiac compression and cardiac asymmetry indexes that focus on cardiac deformity, are potentially valuable tools for preoperative evaluation of PE and for predicting the outcome of repair using the chest wall compression index [5]. Surgical intervention for Poland syndrome is typically reserved for patients with severe rib aplasia and significant depression deformity. Various types of ectopia cordis, including sternal defects, are also discussed [6, 7]. Notably, even after surgical correction, there is a significant decrease in the total lung and inspiratory vital capacities, likely due to reduced chest wall compliance [8].
Over 5% of patients who present with a primary complaint of PD will be diagnosed with Marfan’s syndrome, a figure that stands in contrast to the 0.3% prevalence in the general population [9]. It seems that only around 4% to 5% of individuals with severe anterior CWDs exhibit scoliosis [10]. Patients suffering from PE deformity exhibit varying degrees of right ventricular distortion, which is linked to a 6% decrease in resting right ventricular ejection fraction compared to healthy individuals [11]. For children needing an extracardiac conduit to rectify their congenital heart defect, it is advised to first repair the PE, followed by cardiac lesion repair 6 weeks or later, to prevent potential external compression of the conduit by the indented sternum [12]. Research has uncovered multiple harmful functional and structural heart changes in PE, which include indications of irregular diastolic and systolic function in the right ventricle and constriction of the atrioventricular groove, which intensify under stress and are correlated with the severity of the malformation [13–15].
For many years, an abundance of research has been performed to enhance the quality of care through a deeper comprehension of the etiopathogenesis, epidemiology, occurrence, and clinical characteristics of these deformities. Herein, a systematic review and meta-analysis was designed to assess, for the first time, the frequency and incidence of CWDs in children under the age of 18.
Material and methods
Research design
The meta-analysis was managed following the protocols of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16]. CoCoPop (condition, context, population) framework: What is the prevalence and incidence of CWDs in children in the world? The study has not been registered in any database.
Determination of articles
A comprehensive investigation was conducted by a single author (Y.P.) in the databases of Cochrane Library, PubMed, Scopus, and Web of Science up until March 23, 2024, with an age limitation. The titles and abstracts of the papers were evaluated. Following that, the complete texts of the papers that satisfied the selection criteria were retrieved. The search strategy incorporated: (“pectus anomal*” or “chest anomal*” or “chest wall deformit*” or “pectus excavatum” or “pectus carinatum” or “Poland syndrome” or “sternal cleft” or “ectopia cordis” or “Jeune syndrome” or “Jarcho Levin syndrome” or “keeled chest” or “funnel chest”) and (“prevalen*” or “incidenc*” or “occur*” or “Frequency”) and (“child*” or “pediatric*” or “teenager*” or “adolescent*” or “juvenile” or “infant*”). The bibliographies of the obtained articles were scrutinized to ensure no relevant research was missed. Another author (Y.J.) reconfirmed the search and selection procedure. Any disagreements between the two authors were settled by a third author (W.H.). Since this study is a meta-analysis, it does not require ethical approval.
Eligibility criteria
The criteria for inclusion were as follows: 1) Case-control, cross-sectional, and cohort studies that reported on CWDs. 2) Patients who had not received any treatment or were undergoing surgery. 3) Studies that included cases of individuals under 18 years old. Conversely, review articles, articles with incomplete or no data, animal studies, articles lacking a control group, book chapters, and duplicate studies were omitted.
Data summary
Two authors (Y.P. and Z.Y.) independently gathered the data from the studies that were incorporated into the meta-analysis. The collected data encompassed the first author’s name, the year of publication, the study’s country, the continents where the cases were located, the patient count, and the quality score.
Quality evaluation
One author (L.L.) conducted the quality assessment using the Newcastle-Ottawa Scale (NOS) tool [17]. This tool evaluates a study from three broad perspectives (modified for cross-sectional studies): the selection process of the study groups (5 points), the comparability of the groups (1 point), and the outcome of interest (3 points) for case-control studies. A study with a score of 7 or more is considered high-quality (https://cdn-links.lww.com/permalink/ejgh/a/ejgh_31_9_2019_07_18_nguyen_15743_sdc1.pdf).
Statistical analysis
The Comprehensive Meta-Analysis version 3.0 (CMA 3.0) software was used to compute the effect size, presenting the event rate (ER). In the event that the Pheterogeneity was under 0.1 (I2 > 50%), signifying substantial heterogeneity, a random-effects model [18] was applied. Conversely, if the heterogeneity was not significant, a fixed-effect model [19] was put into use.
A subgroup analysis was conducted based on continents. Subgroup analysis is a method that enables deeper investigation to understand how specific variables influence the result of secondary data analysis. The potential for publication bias was assessed using Begg’s test [20], and the asymmetry level was evaluated using Egger’s test [21]. The p-values were derived from both tests and a two-sided p-value of less than 0.10 suggested the existence of publication bias. In terms of sensitivity analysis, analyses were used to evaluate the robustness and reliability of the combined ER.
Results
Study selection
The total number of identified records was 1,510, comprising 1,508 records identified through main databases and an additional 2 records from other electronic sources (Figure 1). After removing duplicates, 856 records remained for further assessment. Out of the 856 screened records, 49 full-text articles were assessed for eligibility. The flowchart shows that 807 records were excluded during this step, along with 15 full-text articles with the reasons. Finally, 34 articles [9, 12, 22–53] were included in the systematic review and the meta-analysis.
Characteristics of the articles
Table I lists articles included in the meta-analysis. The articles are characterized by the first author and publication year, the country of the study, the number of cases studied, and a quality score for each study. The studies were conducted in various countries, with a significant number from the USA and Turkey. Other countries include Spain, Germany, Brazil, Iran, Korea, China, Thailand, Italy, Denmark, and Poland. The number of cases in these studies varies widely, from as few as 15 cases to as many as 25,117 cases. Some studies specify the type of cases, such as those with PE, PDs, congenital heart disease (CHD), or cancers. The quality score ranges from 6 to 8. Most of the studies have a quality score of ≥ 7, indicating relatively high-quality research. The studies have been published from 1985 to 2023, showing a long-term interest in this research area.
Table I
Characteristics of the articles included in the meta-analysis
| First author, publication year | Country | Number of cases | Quality score |
|---|---|---|---|
| Golladay, 1985 [31] | USA | 42 with pectus excavatum | 6 |
| Shamberger, 1988 [12] | USA | 20860 | 7 |
| Cascos, 1989 [25] | Spain | 2000 with congenital heart disease | 7 |
| Park, 1990 [38] | USA | 87 with pectus deformities | 7 |
| Seliem, 1992 [43] | USA | 31 with pectus excavatum | 7 |
| Yücesan, 1993 [52] | Turkey | 19750 | 8 |
| Donnelly, 1999 [29] | USA | 200 | 7 |
| Mandhan, 1999 [37] | USA | 129 with pectus deformities | 7 |
| Soysal, 1999 [45] | Turkey | 2500 | 8 |
| Trobs, 2001 [48] | Germany | 45 with cancers | 7 |
| Berktafl, 2001 [24] | Turkey | 3183 | 7 |
| Esme, 2006 [30] | Turkey | 3779 | 8 |
| Rhee, 2008 [42] | USA | 37 with pectus excavatum | 7 |
| Westphal, 2009 [51] | Brazil | 1332 | 7 |
| Rajabi-Mashhadi, 2010 [41] | Iran | 13586 | 8 |
| Coskun, 2010 [28] | Turkey | 1342 | 7 |
| Hong, 2011 [32] | Korea | 248 with pectus excavatum | 7 |
| Shu, 2011 [44] | China | 406 with pectus excavatum | 7 |
| Venkatramani, 2013 [49] | USA | 109 with cancers | 7 |
| Tokur, 2016 [46] | Turkey | 25117 | 8 |
| Jantarawan, 2017 [34] | Thailand | 52 with congenital heart disease | 7 |
| Park, 2017 [39] | USA | 468 with pectus deformities | 7 |
| Zhong, 2017 [53] | China | 15 with pectus excavatum | 7 |
| Tomaszewski, 2017 [47] | Poland | 54 with pectus excavatum | 7 |
| Akkaş, 2018 [23] | Turkey | 14108 | 8 |
| Behr, 2019 [9] | USA | 241 with pectus deformities | 7 |
| Port, 2020 [40] | USA | 155 with pectus carinatum | 7 |
| Chelleri, 2021 [26] | Italy | 693 with cancers | 7 |
| Christensen, 2021 [27] | Denmark | 1046 with pectus excavatum | 7 |
| Wang, 2022 [50] | China | 256 with pectus excavatum | 7 |
| Acipayam, 2023 [22] | Turkey | 36 with pectus excavatum | 8 |
| Katrancioglu. 2023 [36] | Turkey | 15862 | 8 |
| Karabulut, 2023 [35] | Turkey | 109 with pectus deformities | 8 |
| Hou, 2023 [33] | China | 575 with pectus excavatum | 6 |
Pooled ERs
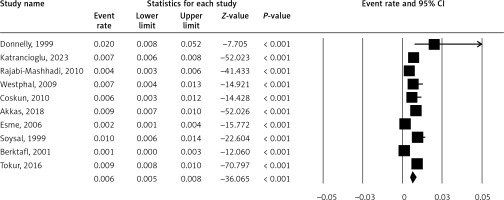
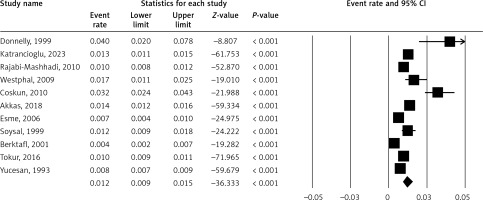
Figure 2 shows the prevalence of PE, a condition where the sternum sinks into the chest. The pooled ER is 0.5% (5% CI: 0.3–0.9%). A high I2 value (91.41%) may suggest substantial heterogeneity.
Figure 3 shows the prevalence of PC, a condition where the sternum protrudes. The pooled ER is 0.6% (5% CI: 0.5–0.8%). A high I2 value (85.93%) may suggest substantial heterogeneity.
Figure 4 shows the prevalence of PDs. The pooled ER is 1.2% (5% CI: 0.9–1.5%). A high I2 value (92.26%) may suggest substantial heterogeneity.
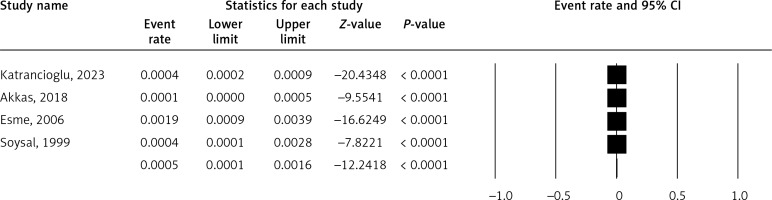
Figure 5 shows the prevalence of rib anomaly. The pooled ER is 0.05% (5% CI: 0.01–0.16%). A high I2 value (78.02%) may suggest substantial heterogeneity.
Figure 6 shows the Marfan’s syndrome incidence in PDs (I2 = 91.21%) and the mitral valve prolapse (MVP) incidence in PDs. The pooled ER is 11.4% (5% CI: 3.5–31.4%) for the Marfan’s syndrome incidence in PDs and 19.7% (5% CI: 10.2–34.7%) for the MVP incidence in PDs.
Figure 6
A – Incidence of Marfan’s syndrome in pectus deformities (I2 = 91.21%). B – Incidence of mitral valve prolapse in pectus deformities (I2 = 92.19%)
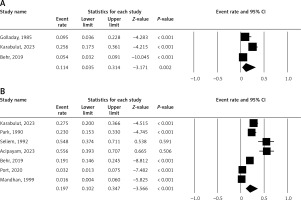
Figure 7 shows the incidence of CWDs in cancers (I2 = 82.50%), the incidence of PDs in CHD (I2 = 99.40%, and the scoliosis incidence in PE (I2 = 91.41%). The pooled ER is 6.2% (5% CI: 2.8–14.1%) for the incidence of CWDs in cancers, 2.6% (5% CI: 0.1–32.9%) for the incidence of PDs in CHD, and 16% (5% CI: 9.5–25.7%) for the incidence of scoliosis in PE.
Figure 7
A – Incidence of chest wall deformities in cancers (I2 = 82.50%). B – Incidence of pectus deformities in congenital heart disease (I2 = 99.40%). C – Incidence of scoliosis in pectus excavatum (I2 = 91.41%)
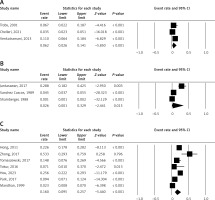
Figure 8 shows the incidence of cardiac anomalies in PDs (I2 = 98.19%), the incidence of abnormal electrocardiogram in PDs, the incidence of aortic root dilation in PDs (I2 = 76.30%), the tricuspid valve prolapse (TVP) incidence in PDs, and the CHD incidence in PE (I2 = 00.00%). The pooled ER is 11.1% (5% CI: 3.0–33.3%) for the incidence of cardiac anomalies in PDs, 13.4% (5% CI: 6.2–26.9%) for the incidence of abnormal electrocardiogram in PDs, 12.6% (5% CI: 6.8–22.2%) for the incidence of aortic root dilation in PDs, 6.3% (5% CI: 0.3–62.3%) for the incidence of TVP in PDs, and 2% (5% CI: 1.2–3.4%) for the incidence of CHD in PE.
Figure 8
A – Incidence of cardiac anomalies in pectus deformities (I2 = 98.19%). B – Incidence of abnormal electrocardiogram in pectus deformities. C – Incidence of aortic root dilation in pectus deformities (I2 = 76.30%). D – Incidence of tricuspid valve prolapse in pectus deformities. E – Incidence of congenital heart disease in pectus excavatum (I2 = 00.00%)

Subgroup analysis
A subgroup analysis was done based on continent (Table II). The result showed that the prevalence of PE, the prevalence of PC, and the prevalence of PDs in Asian individuals were higher than in American individuals. In addition, the MVP incidence in PDs and the incidence of scoliosis in PE in American individuals were higher than in Asian individuals.
Table II
Subgroup analysis
Sensitivity analysis
Both “cumulative” and “one-study-removed” analyses recommended the stability of the pooled results.
Publication bias
Neither Egger’s nor Begg’s tests indicated any publication bias among the studies (p > 0.10). The Supplementary Figures S1–S12 present the funnel plots for the analyses that included at least three studies.
Discussion
The study discovered that a significant proportion (44%) of patients who underwent surgical correction for PE had a history of comorbidities and previous medical conditions. There was a noticeable prevalence of conditions such as hernias, allergies, asthma, psychiatric disorders, and past polyp-/tonsillectomies (27), as well as cardiac abnormalities, Marfan’s syndrome (9), scoliosis, cancers (27), obstructive sleep apnea, and pulmonary blebs [54, 55]. Children with a family history of CWDs tend to have a higher awareness of the deformity, especially in more insular societies. This is because the knowledge of the disease is already present within the family [23].
This present systematic review and meta-analysis presents various figures showing the prevalence and incidence of different CWDs and related conditions. PE and PC have a prevalence of 0.5% and 0.6%, respectively, while PDs overall have a prevalence of 1.2%. Rib anomalies are less common with a prevalence of 0.05%. The incidence of Marfan’s syndrome and MVP in PDs is 11.4% and 19.7% respectively. The study also shows the incidence of CWDs in cancers (6.2%), PDs in CHD (2.6%), and scoliosis in PE (16%). Cardiac anomalies, abnormal electrocardiograms, aortic root dilation, TVP, and CHD in PE have incidences of 11.1%, 13.4%, 12.6%, 6.3%, and 2% respectively. A subgroup analysis showed a higher prevalence of PDs in Asian individuals compared to American individuals, but a higher incidence of MVP and scoliosis in American individuals. The results were found to be stable and no publication bias was detected.
In younger patients, the thoracic wall’s flexibility allows the heart to shift to the left, reducing heart compression somewhat [22]. However, as individuals age, the thoracic wall loses its flexibility, becomes stiffer, and the heart’s leftward shift decreases, leading to increased heart compression and symptoms [56]. A considerable number of PE patients show one or more functional and/or morphological changes in the heart [15]. Moreover, the extent of these changes correlates with chest deformation indexes and the cardiac compression classification, which pinpoints the maximum compression location on the right heart chambers [15]. Numerous studies in the literature have reported that CWDs impact cardiopulmonary functions. It has been reported that cardiopulmonary functions improve after surgery [57, 58].
A study conducted on a large Turkish population revealed that CWDs were more prevalent in boys (0.96%), with PC being the most common deformity [23]. It is well known that CWDs and scoliosis often occur together. Westphal et al. reported a 15% co-occurrence rate among Brazilian students, but this study found a lower rate of 5.03% [51]. Despite this, no link was discovered between the severity of the deformity and scoliosis, a conclusion also drawn by Frick in their review [10]. This discrepancy could be due to the absence of simultaneous inspection and radiological assessment in the studies.
In a comprehensive, single-center review spanning from 1987 to 2010, Kelly et al. found that 2.8% of patients who underwent surgical correction for PE were genetically diagnosed with Marfan’s syndrome, and an additional 17% exhibited clinical signs indicative of Marfan’s syndrome [59]. Numerous studies that explore the connections between PE, Marfan’s syndrome, and cardiovascular defects primarily focus on patients who have undergone surgical repair for excavatum [9]. Cardiovascular defects linked to Marfan’s syndrome are the primary contributors to morbidity and mortality in these patients [60, 61]. Furthermore, 75% of patients with Marfan’s syndrome experience MVP, which can lead to complications such as mitral valve regurgitation [60, 61].
Limitations: 1) The small numbers of studies published for each analysis, which meant that we had an incomplete subgroup analysis and no meta-regression analysis. 2) High heterogeneity between the studies and therefore the results should be interpreted with caution. Strengths: 1) The stability of the pooled results. 2) Lack of publication bias. 3) Having high quality in most studies.
In conclusion, this comprehensive meta-analysis provides a detailed overview of the prevalence and incidence of various CWDs and associated conditions. The results indicate a substantial heterogeneity across the studies, suggesting the need for further research to understand the underlying causes of these variations. The study also highlights the higher prevalence of PDs in Asian individuals compared to American individuals, and a higher incidence of certain conditions such as MVP and scoliosis in PE in American individuals.
The findings of this study have significant clinical implications. Understanding the prevalence and incidence of these deformities can help in early detection and treatment, potentially improving the quality of life for affected individuals. Moreover, the higher incidence of certain conditions in individuals with PDs underscores the importance of regular monitoring and comprehensive care for these patients.
In future, it would be beneficial to conduct more region-specific studies to understand the factors contributing to the observed geographical differences in the prevalence of these deformities. Additionally, longitudinal studies could provide insights into the long-term outcomes and effectiveness of various treatment strategies for these conditions. The stability of the pooled results, as indicated by the “cumulative” and “one-study-removed” analyses, provides a solid foundation for future research in this area. Finally, the absence of publication bias, as indicated by both Egger’s and Begg’s tests, adds to the credibility of these findings and underscores the need for continued research in this field.