Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
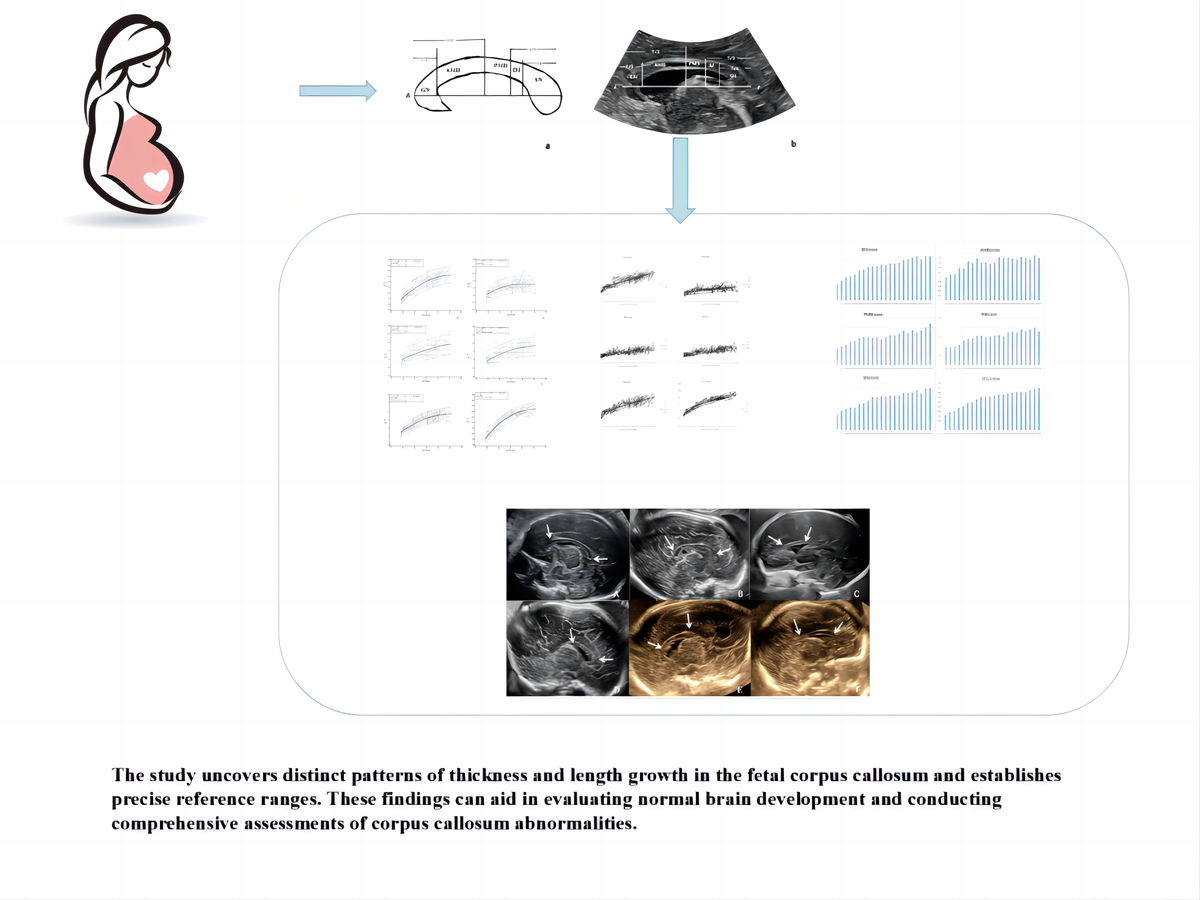
Prenatal ultrasound reassessment of the corpus callosum based on cortical connectivity information
1
Department of Radiology, The Second Affiliated Hospital of Shandong First Medical University, Taian, China
2
Department of Radiology, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, China
3
Department of Ultrasound, Weifang Hospital of Traditional Chinese Medicine, Weifang, China
4
Department of Ultrasound, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, China
5
Department of Ultrasound, Shandong Provincial Qingzhou People’s Hospital, Weifang, China
These authors had equal contribution to this work
Submission date: 2023-11-08
Final revision date: 2023-12-25
Acceptance date: 2024-01-02
Online publication date: 2024-05-28
Corresponding author
Jian Qin
Department of Radiology The Second Affiliated Hospital of Shandong First Medical University Taian, China
Department of Radiology The Second Affiliated Hospital of Shandong First Medical University Taian, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Routine prenatal ultrasound assessment of the corpus callosum (CC) does not reflect information on fibrous connections. The primary purpose was to construct detailed reference ranges of quantitative characteristics of the foetal CC based on cortical connectivity information. Secondary goals were to examine for sex differences and assess the validity of the measurement technique for cases with CC dysplasia.
Material and methods:
Pregnant women referred to a tertiary centre for sonographic examination were recruited to undergo a detailed foetal scan from 19 to 40 weeks. The foetal CC was divided into 5 distinct segments using the Hofer & Frahm classification technique. The thickness of each segment and the overall length of the CC were measured. Additionally, a segmental evaluation was conducted on partial agenesis of the CC. The inter- and intraobserver variability were assessed by interclass correlation coefficients. Regression analysis was used to determine the association between the biological measurements and gestational age.
Results:
A total of 852 foetuses (403 males and 449 females) were included in the final analysis. Intra- and interobserver reliability coefficients ranged from 0.86 to 0.98 and 0.84 to 0.97, respectively. Reference ranges were established for the thickness and length of its segments. We observed that the biometric measurements of the foetal CC showed a curvilinear increase with gestational age. There was a statistically significant sex effect for the CC. At the average gestation age 29.6 weeks, the genu, anterior midbody, posterior midbody, and isthmus of male foetuses were 0.06280 mm, 0.04435 mm, 0.01731 mm, and 0.01556 mm, respectively, thicker than those of female foetuses, whereas the splenium of the female foetus was 0.06583 mm thicker than the male foetus.
Conclusions:
The study uncovers distinct patterns of thickness and length growth in the foetal CC and establishes precise reference ranges. These findings can aid in evaluating normal brain development and conducting comprehensive assessments of CC abnormalities.
Routine prenatal ultrasound assessment of the corpus callosum (CC) does not reflect information on fibrous connections. The primary purpose was to construct detailed reference ranges of quantitative characteristics of the foetal CC based on cortical connectivity information. Secondary goals were to examine for sex differences and assess the validity of the measurement technique for cases with CC dysplasia.
Material and methods:
Pregnant women referred to a tertiary centre for sonographic examination were recruited to undergo a detailed foetal scan from 19 to 40 weeks. The foetal CC was divided into 5 distinct segments using the Hofer & Frahm classification technique. The thickness of each segment and the overall length of the CC were measured. Additionally, a segmental evaluation was conducted on partial agenesis of the CC. The inter- and intraobserver variability were assessed by interclass correlation coefficients. Regression analysis was used to determine the association between the biological measurements and gestational age.
Results:
A total of 852 foetuses (403 males and 449 females) were included in the final analysis. Intra- and interobserver reliability coefficients ranged from 0.86 to 0.98 and 0.84 to 0.97, respectively. Reference ranges were established for the thickness and length of its segments. We observed that the biometric measurements of the foetal CC showed a curvilinear increase with gestational age. There was a statistically significant sex effect for the CC. At the average gestation age 29.6 weeks, the genu, anterior midbody, posterior midbody, and isthmus of male foetuses were 0.06280 mm, 0.04435 mm, 0.01731 mm, and 0.01556 mm, respectively, thicker than those of female foetuses, whereas the splenium of the female foetus was 0.06583 mm thicker than the male foetus.
Conclusions:
The study uncovers distinct patterns of thickness and length growth in the foetal CC and establishes precise reference ranges. These findings can aid in evaluating normal brain development and conducting comprehensive assessments of CC abnormalities.
REFERENCES (31)
1.
Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec 1954; 119: 119-35.
2.
Paul LK, Van Lancker-Sidtis D, Schieffer B, et al. Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain Lang 2003; 85: 313-24.
3.
Clarke JM, Zaidel E. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res 1994; 64: 185-202.
4.
Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 1989; 112: 799-835.
5.
Duara R, Kushch A, Gross-Glenn K, et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch Neurol 1991; 48: 410-6.
6.
Rajapakse JC, Giedd JN, Rumsey JM, et al. Regional MRI measurements of the corpus callosum: a methodological and developmental study. Brain Dev 1996; 18: 379-88.
7.
Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006; 32: 989-94.
8.
Liang Y, Shao R, Zhang Z, Li X, Zhou L, Guo S. Amplitude of low-frequency fluctuations in childhood-onset schizophrenia with or without obsessive-compulsive symptoms: a resting-state functional magnetic resonance imaging study. Arch Med Sci 2019; 15: 126-33.
9.
Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 2010; 52: 447-77.
10.
Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Arch Neurol 1992; 49: 271-7.
11.
Arda KN, Akay S. The relationship between corpus callosum morphometric measurements and age/gender characteristics: a comprehensive MR imaging study. J Clin Imaging Sci 2019; 9: 33.
12.
Leonard CM, Towler S, Welcome S, et al. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb Cortex 2008; 18: 2920-31.
13.
Joseph JE, Willingham DB. Effect of sex and joystick experience on pursuit tracking in adults. J Mot Behav 2000; 32: 45-56.
14.
Sonographic examination of the fetal central nervous system: guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol 2007; 29: 109-16.
15.
Pashaj S, Merz E, Wellek S. Biometry of the fetal corpus callosum by three-dimensional ultrasound. Ultrasound Obstet Gynecol 2013; 42: 691-8.
16.
Achiron R, Achiron A. Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol 2001; 18: 343-7.
17.
Goldstein I, Tamir A, Reece AE, et al. Corpus callosum growth in normal and growth-restricted fetuses. Prenat Diagn 2011; 31: 1115-9.
18.
Araujo Júnior E, Visentainer M, Simioni C, et al. Reference values for the length and area of the fetal corpus callosum on 3-dimensional sonography using the transfrontal view. J Ultrasound Med 2012; 31: 205-12.
19.
Malinger G, Zakut H. The corpus callosum: normal fetal development as shown by transvaginal sonography. AJR Am J Roentgenol 1993; 161: 1041-3.
20.
Karl K, Esser T, Heling KS, et al. Cavum septi pellucidi (CSP) ratio: a marker for partial agenesis of the fetal corpus callosum. Ultrasound Obstet Gynecol 2017; 50: 336-41.
21.
Cignini P, Padula F, Giorlandino M, et al. Reference charts for fetal corpus callosum length: a prospective cross-sectional study of 2950 fetuses. J Ultrasound Med 2014; 33: 1065-78.
22.
Harreld JH, Bhore R, Chason DP, et al. Corpus callosum length by gestational age as evaluated by fetal MR imaging. AJNR Am J Neuroradiol 2011; 32: 490-4.
23.
Achiron R, Lipitz S, Achiron A. Sex-related differences in the development of the human fetal corpus callosum: in utero ultrasonographic study. Prenat Diagn 2001; 21: 116-20.
24.
Hwang SJ, Ji EK, Lee EK, et al. Gender differences in the corpus callosum of neonates. Neuroreport 2004; 15: 1029-32.
25.
Tilea B, Alberti C, Adamsbaum C, et al. Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol 2009; 33: 173-81.
26.
Koshi R, Koshi T, Jeyaseelan L, et al. Morphology of the corpus callosum in human fetuses. Clin Anat 1997; 10: 22-6.
27.
Rosenbloom JI, Yaeger LH, Porat S. Reference ranges for corpus callosum and cavum septi pellucidi biometry on prenatal ultrasound: systematic review and meta-analysis. J Ultrasound Med 2022; 41: 2135-48.
28.
Tsur A, Weisz B, Rosenblat O, et al. Personalized charts for the fetal corpus callosum length. J Matern Fetal Neonatal Med 2019; 32: 3931-8.
29.
Miguelote RF, Vides B, Santos RF, Palha JA, Matias A, Sousa N. The role of three-dimensional imaging reconstruction to measure the corpus callosum: comparison with direct mid-sagittal views. Prenat Diagn 2011; 31: 875-80.
30.
Rizzo G, Pietrolucci ME, Capponi A, et al. Assessment of corpus callosum biometric measurements at 18 to 32 weeks’ gestation by 3-dimensional sonography. J Ultrasound Med 2011; 30: 47-53.
31.
Chang CL, Chiu NC, Yang YC, et al. Normal development of the corpus callosum and evolution of corpus callosum sexual dimorphism in infancy. J Ultrasound Med 2018; 37: 869-77.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.