Introduction
Traumatic brain injury (TBI) has a high rate of disability and fatality, and the incidence of TBI in China is about 0.2% [1]. Severe TBI accounted for about 20% of TBI patients, among whom the fatality rate of severe craniocerebral injury was 36.8–68.3% [2, 3]. An individual was deemed to have hyperglycemia if two distinct instances of random blood glucose tests showed a fasting glucose concentration above 6.1 mmol/l or a casual glucose concentration that exceeded 11.1 mmol/l [4]. After severe TBI, hyperfunction of the sympathetic-adrenal system is caused by excitation of the hypothalamus-pituitary-target gland axis, which leads to the increase of blood glucose [5, 6]. Some studies [7, 8] have found that the increase of blood glucose in patients with severe TBI is related to the degree and duration of disturbance of consciousness. In recent years, a large number of animal experiments and clinical studies have confirmed that the increase of blood glucose aggravates the secondary brain damage, and blood glucose is related to the prognosis of severe TBI [9, 10]. Therefore, the blood glucose management of patients with TBI is of great significance for the prognosis of patients.
It is currently recognized that the stress induced by TBI triggers a cascade of neuroendocrine responses. Activation of the sympathetic nervous system and the adrenal medulla, along with increased secretion of hormones such as growth hormone, glucagon, adrenocorticotropin, and thyrotropin, can contribute to elevated blood glucose levels. These hormonal changes are part of the body’s stress response, which, in the context of TBI, can have significant implications for glucose homeostasis and overall patient management [11, 12]. Although hyperglycemia in critically ill patients is transient, if not treated in time, it will cause serious damage to the body and affect the outcome of the disease [13–16]. At present, blood glucose is one of the important factors that affect the prognosis of severe TBI. The longer the duration of hyperglycemia is, the worse is the prognosis [17, 18]. Blood glucose monitoring of patients with TBI has the characteristics of accurate, rapid, simple, easy and low cost for judging injury condition and prognosis [19]. It is an important means to monitor, guide treatment and judge prognosis of patients with TBI. The aim of this study was to conduct a comprehensive review and analysis of the prevailing conditions and contributing factors associated with hyperglycemia in patients with TBI within the initial 48 h following surgical intervention. Furthermore, the study sought to create a predictive model for postoperative hyperglycemia specifically tailored to TBI patients. This model is intended to serve as a vital source of evidence-based support, informing and enhancing the treatment and nursing care strategies for individuals affected by TBI.
Material and methods
Ethics
This investigation was conducted as a retrospective cohort study focused on the status quo and factors influencing postoperative hyperglycemia in patients with TBI. The research protocol was reviewed and approved by the Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, with the assigned approval number 20220046. All data collected were utilized solely for research purposes, ensuring the privacy and ethical treatment of the study participants.
Study population
In this study, we included patients who experienced TBI and subsequently underwent craniocerebral surgery at our facility between March 1, 2022, and October 31, 2023. The inclusion criteria were defined as follows: adult patients with age ≥ 18 years old; the patient had a clear history of brain trauma and was admitted to hospital within 8 h after injury, and was diagnosed with TBI by brain CT scan [20]; the patients underwent surgery treatment in our hospital. The exclusion criteria were as follows: Patients with bilateral dilated pupils and dying TBI patients; TBI patients referred to our hospital from other hospitals; TBI patients with incomplete treatment data.
Data collection
The diagnosis of postoperative hyperglycemia was established based on the following criteria: a patient was considered to have hyperglycemia if two separate random blood glucose measurements revealed a fasting blood glucose level greater than 6.1 mmol/l or a random blood glucose level exceeding 11.1 mmol/l [21]. Blood glucose levels were closely monitored every 3 h for the first 48 h postoperatively. Once a TBI patient was diagnosed with hyperglycemia, they were promptly initiated on a standard insulin therapy regimen. Subsequently, the following data were meticulously gathered from the patients’ medical and nursing record: age, gender, body mass index (BMI), hypertension, diabetes, hyperlipidemia, Glasgow Coma Scale (GCS) score, duration of operation, length of hospital stay.
Statistical analysis
All collected data underwent analysis using the SPSS 23.0 software package. The measured data were presented as the mean ± standard deviation, with t-tests and analysis of variance (ANOVA) applied to analyze these data sets. Categorical data were assessed using the chi-square (χ2) test, while Pearson’s correlation analysis was employed to examine the characteristics of the included TBI patients in relation to hyperglycemia. To pinpoint the independent factors contributing to postoperative hyperglycemia among TBI patients, logistic regression analysis was conducted. Scores were allocated to significant factors based on their respective partial regression coefficients in the logistic regression model. Subsequently, a risk prediction model for postoperative hyperglycemia in TBI patients was developed. The model’s goodness-of-fit was evaluated using the Hosmer-Lemeshow (H-L) test. The sensitivity and specificity of the risk model were determined using the area under the receiver operating characteristic (ROC) curve. In this study, a difference was considered statistically significant if the p-value was less than 0.05.
Results
A total of 216 TBI patients were included in this study, of whom 68 patients had hyperglycemia within 48 h after the operation. The incidence of postoperative hyperglycemia in TBI patients was 31.48% in this study. As shown in Table I, statistically significant differences in the age, BMI, diabetes, GCS score and length of hospital stay were found between hyperglycemia and no hyperglycemia patients with TBI (all p < 0.05). No significant differences in gender, hypertension, hyperlipidemia and duration of operation were observed between hyperglycemia and no hyperglycemia patients with TBI (all p > 0.05).
Table I
Characteristics of included TBI patients
As indicated in Table II, results of correlation analysis indicated that age (r = 0.415), BMI (r = 0.441), diabetes (r = 0.513), GCS score (r = 0.545) and length of hospital stay (r = 0.456) were all correlated with the occurrence of postoperative hyperglycemia in TBI patients (all p < 0.05).
Table II
Correlation analysis on the characteristics of included TBI patients and hyperglycemia
The length of hospital stay was the result of hyperglycemia, so it was not taken as an independent variable in logistics regression analysis. This study included the other variables with statistical difference in the univariate analysis in further logistic regression analysis. The variable assignment for multivariate logistic regression analysis in this study is presented in Table III. As presented in Table IV, the findings from the logistic regression analysis revealed that age ≥ 60 years (OR = 2.556, 95% CI: 1.831–3.641), BMI ≥ 24 kg/m2 (OR = 2.793, 95% CI: 2.305–3.679), diabetes (OR = 3.081, 95% CI: 2.326–3.811) and GCS score ≤ 8 (OR = 3.603, 95% CI: 1.956–4.086) were significant independent risk factors for postoperative hyperglycemia in TBI patients (all p < 0.05).
Table III
Variable assignment for multivariate logistic regression analysis
| Factors | Variables | Assignment |
|---|---|---|
| Hyperglycemia | Y | Yes = 1, no = 2 |
| Age [years] | X1 | ≥ 60 = 1, < 60 = 2 |
| BMI [kg/m2] | X2 | ≥ 24 = 1, < 24 = 2 |
| Diabetes | X3 | Yes = 1, no = 2 |
| GCS score | X4 | ≤ 8 = 1, > 8 = 2 |
Table IV
Logistic regression analysis on the factors influencing hyperglycemia in TBI patients
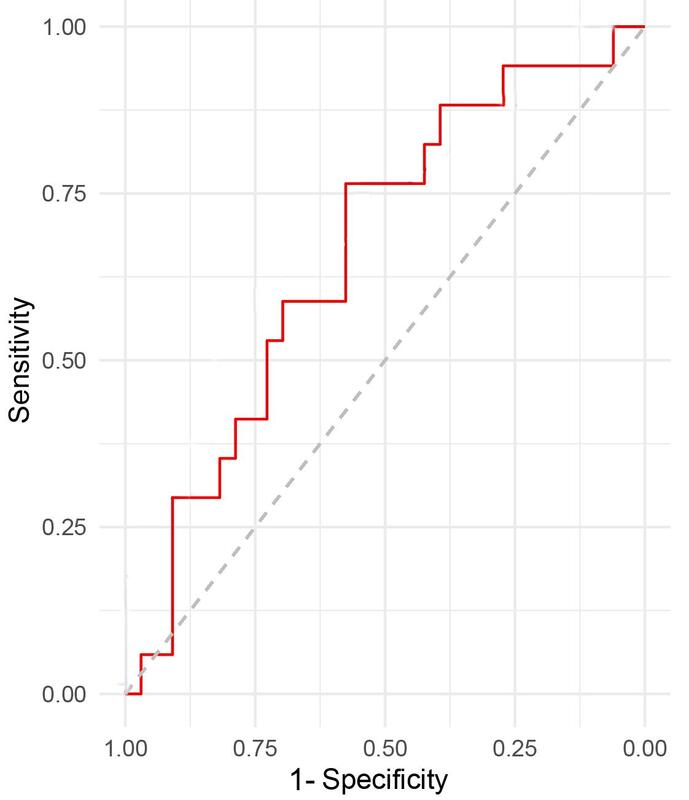
As shown in Table V, this study developed the scoring criteria for a predictive model of postoperative hyperglycemia in TBI patients. Based on the ROC curve (Figure 1) and scoring standard of the risk prediction model, the sensitivity and specificity of the prediction model under different cut-off scores were calculated and compared, and the relevant Youden index (sensitivity + specificity –1) was calculated. As presented in Table VI, the Youden index was higher when the total score was between 5.5 and 6.5. Thus the total score = 6 was used as the cut-off value of this risk prediction model. The sensitivity and specificity of the prediction model were all high when the total score = 6. The area under the ROC curve (AUC) and 95% CI were 0.795 (0.712, 0.849), indicating that the risk prediction model had good discriminative ability to distinguish the occurrence of postoperative hyperglycemia in TBI patients (all p < 0.05).
Table V
Scoring method of the logistic model for the risk of hyperglycemia in TBI patients
| Variable | Score |
|---|---|
| Age ≥ 60 years | 2 |
| BMI ≥ 24 kg/m2 | 2 |
| Diabetes | 3 |
| GCS score ≤ 8 | 3 |
Table VI
Sensitivity and specificity of the prediction model under different cut-off values
Discussion
Hyperglycemia represents a metabolic response of the body following traumatic or stressful events. While it may be transient in nature, failure to address it promptly can lead to significant harm to the body and adversely impact the disease’s prognosis [22–24]. A number of previous studies [25–27] have confirmed that hyperglycemia has an adverse effect on the prognosis of critically ill patients. It has been reported that the control of hyperglycemia will be beneficial to the prognosis of TBI [28]. The results of this study show that the incidence of postoperative hyperglycemia in TBI patients is 31.48%, and age ≥ 60 years, BMI ≥ 24 kg/m2, diabetes and GCS score ≤ 8 are the factors independently influencing postoperative hyperglycemia in TBI patients. Additionally, this study has developed a useful prediction model for postoperative hyperglycemia in TBI patients, which is easy and helpful for the clinical treatment and nursing care of TBI patients.
Hyperglycemia is very common in postoperative patients. There is a strong stress response after craniocerebral injury, which leads to over-excitation of the sympathetic-adrenal medulla system and the increase of catecholamine in the blood [29, 30]. The sympathetic efferent pathway of the hypothalamus and brainstem is damaged by primary or secondary factors, which excites the sympathetic nerve and leads to a sharp increase in the secretion of catecholamine in the body, stimulating the release of glucagon, decomposing liver glycogen and inhibiting insulin secretion [31–33]. On the one hand, the increase of blood glucose after TBI strengthens glycolysis and the production of lactic acid, which leads to a large number of Na+ ions entering the cell and induces cellular brain edema [34]. On the other hand, the strengthening of anaerobic metabolism can lead to the disturbance of energy generation, resulting in intracellular high sodium, extracellular high potassium and calcium overload, increasing the catabolism of adenosine 5′-triphosphate, cellular protein and lipid, and damaging the cellular skeleton system and membrane system [35, 36]. All of the above factors can further aggravate the degree of intracellular brain edema and then aggravate the increase of intracranial pressure after TBI [37, 38]. If it cannot be effectively controlled, it will fall into a vicious circle of intracranial hypertension, brain edema, cerebral hypoxia and aggravated intracranial hypertension, resulting in the death of patients [39–41]. Therefore, dynamic monitoring of blood glucose and active control of blood glucose level has become one of the keys to the treatment of TBI patients.
Hyperglycemia has received more and more attention in clinical work. Hyperglycemia will prolong the wound recovery time and hospitalization time of patients, and delay the time of early recovery of patients [42]. At present, under the advocacy of the concept of accelerated rehabilitation surgery, higher requirements and standards have been put forward for the treatment and nursing of TBI patients. Currently most hospitals still use venous blood or peripheral blood to monitor patients’ blood glucose level, and use combined intravenous infusion of insulin to treat the hyperglycemia response in critically ill patients [43]. However, frequent blood collection and simple peripheral or venous blood collection bring discomfort to patients, at the same time, they only reflect the instantaneous blood glucose level at a fixed time point, and cannot identify abnormal blood glucose in real time [44, 45]. Many studies [46–48] have shown that blood glucose fluctuation is one of the important factors affecting the prognosis of patients with severe diseases. One study [49] used a dynamic blood glucose monitoring system, through blood glucose monitoring at as many as 288 time points a day; the results revealed a correlation between blood glucose fluctuation and the condition of patients with craniocerebral trauma. Therefore, continuous monitoring and management of blood glucose in patients with TBI has an important impact on the prognosis of patients. The predictive model for postoperative hyperglycemia in TBI patients developed in this study exhibits good fit, as evidenced by the H-L test and the ROC curve analysis. These results confirm that the model possesses high predictive accuracy and discriminative power. Patients with a total score of 6 or higher are at an increased risk for the development of postoperative hyperglycemia. Consequently, prompt recognition and vigilant nursing care are essential to reduce the incidence of postoperative hyperglycemia in this patient population. It is necessary to continuously observe the relationship between the use of hypoglycemic drugs and the fluctuation of blood glucose, keep blood glucose in a safe range, detect abnormalities as soon as possible and deal with them, and reduce secondary brain damage to a minimum, so as to improve the quality of life of patients with brain trauma [50, 51].
Several limitations of this study must be considered. Firstly, this was a single-centered study, with a small sample size, so it may be underpowered to detect the factors potentially associated with postoperative hyperglycemia. Secondly, due to the retrospective nature of this study design, there may exist additional factors that could influence the development of postoperative hyperglycemia which were not accounted for in the analysis. Thirdly, this study lacks an assessment of glycated hemoglobin, or glycated albumin, to correct patients’ recent blood sugar levels. In clinical practice, the combination of glycated hemoglobin and glycated albumin can more accurately reflect the changes of blood glucose control in patients. The standardization of detection methods and indicators, as well as the development of portable devices, can help diabetic patients better understand their blood glucose control level and achieve better blood sugar control. Finally, the prediction model for postoperative hyperglycemia in TBI patients in this study needs to be verified by more prospective studies with larger sample size in the future. Our research team will expand the sample size and increase the number of positive samples in the future to further verify the predictive performance of this risk model, to provide useful and reliable evidence for clinical TBI treatment and care.
In conclusion, this study found that the incidence of postoperative hyperglycemia in TBI patients was 31.48%, and TBI patients with age ≥ 60 years, BMI ≥ 24 kg/m2, diabetes and GCS score ≤ 8 have higher risk of postoperative hyperglycemia. Furthermore, this study has developed a useful predictive model for postoperative hyperglycemia in TBI patients. The model is easy and convenient for clinical use, which is helpful for the risk evaluation of TBI patients.