Atherosclerosis constitutes a chronic and multifactorial disease associated with high mortality rates globally. A key element initiating the atheromatous process is the oxidative modification of low-density lipoprotein (LDL), which leads to the formation of MM-LDL particles (minimally modified LDL, minimally oxidised LDL) [1, 2]. The oxidative modification of LDL is inhibited in part by the enzymatic activity of proteins contained in high-density lipoprotein (HDL) molecules. One such protein is paraoxonase 1 (PON1, aryldialkylphosphatase, EC 3.1.8.1). The anti-atherosclerotic action of PON1 may result not only from inhibition of oxidative modification of the lipids and their accumulation in LDL particles, but also from hydrolysis of homocysteine thiolactone: a homocysteine derivative that is an independent risk factor for cardiovascular diseases in humans [3].
PON1 is produced in the liver and secreted into the bloodstream, where it binds to HDL. A key amino acid is encoded in position 192 of PON1; this can be either glutamine, encoded by the Q allele, or arginine, by the R allele. The presence of glutamine appears to be related with greater protection against oxidation [4]. The amino acid in position 192 is a part of the active centre, and anchors PON1 in the HDL particle. As such, the Q192R polymorphism (rs662) affects the substrate specificity of PON1, and the 192QQ genotype leads to the greatest capability for preventing LDL from oxidation [5].
The present study examines the role of the Q192R polymorphism as an indicator of the predisposition to atherosclerosis requiring percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) in the Polish population.
Methods
This retrospective study was performed in 282 individuals of both sexes. All the participants underwent coronarography in two Polish centres according to guidelines of the European Society of Cardiology (ESC) [6–9]. This research was conducted in compliance with the provisions included in the Declaration of Helsinki and its plan was approved by the Bioethics Committee (opinions no. 43/2016 and 63/2019). The study group involved 140 patients (57 women and 83 men) after PCI with stent implantation or patients qualified for CABG, with stenoses of minimum 70%. In the control group, there were 142 patients (78 women and 64 men) after coronarography and without essential lesions in coronary vessels. The median age of the study group patients (65.7 yrs.; range: 45.0–88.0 yrs.) was similar to the median in the control group (64.3 yrs.; range: 40.0–89.0 yrs.). Most patients were treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), which may constitute a major confounder in this study. The inclusion criteria included: qualification for coronary angiography, age of at least 18, and obtaining informed consent. The only exclusion criterion was previous dissection of a coronary vessel.
Genomic DNA was extracted from peripheral blood leukocytes using the GeneMATRIX Quick Blood DNA Purification Kit (EURX Ltd., Molecular Biology Products, Poland). The determination of the Q192R PON1 polymorphism was performed with the PCR-RFLP method developed by Arpaci et al. [10]. The PON1 fragment amplicons were digested with the restriction enzyme Alw1 (New England BioLabs, Inc.), and separated in 3% agarose gel afterwards. The obtained bands, reflecting the DNA fragments, were stained with ethidium bromide and visualised under UV light.
Statistical analysis
Calculations were made with STATISTICA 13.3 (data analysis software system, StatSoft Poland). The differences in the determined frequencies were evaluated using the χ2 test. Normal distribution of the continuous variables and the homogeneity of variance between the groups was confirmed with the Shapiro-Wilk W-test and the Brown-Forsythe test, respectively. Where a given variable was not normally distributed or homogeneity of variance lacked, the non-parametric Mann-Whitney U-test was used for comparison. When normal distribution and homogeneity of variance were present, the t-test for independent samples was used. Values of p < 0.05 were considered as statistically significant.
Results
There were no statistically significant differences in terms of medians of individual lipid profile parameters between the study group and the control group: total cholesterol – 162.5 mg/dl vs. 171.0 mg/dl, LDL-C – 80.0 mg/dl vs. 84.0 mg/dl, triglycerides (TG) – 132.0 mg/dl vs. 110.0 mg/dl, non-HDL-C – 107 mg/dl vs. 112.2 mg/dl. A statistically significant difference was found only in the case of median HDL-C: 53.0 mg/dl vs. 58.0 mg/dl (p = 0.023). The analyses of PON1 genotypes and alleles also with regard to parameters of the lipid profile were performed. Their results were presented in Tables I and II.
Table I
PON1 genotypes for the Q192R polymorphism and parameters of the lipid profile
| Components of the lipid profile | Patients after PCI or qualified for CABG (the study group), n = 140 | Patients with no significant lesions in coronary arteries (the control group), n = 142 | Statistical significance of differences between the groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QR | RR | QR | RR | QR | RR | ||||
| n (%) OR 95% CI | 56 (40%) | 40 (28.6%) | 44 (31.4%) | 87 (61.3%) | 33 (23.2%) | 22 (15.5%) | 0.42 0.26-0.68 p = 0.0004* | 1.32 0.77-2.26 p = 0.307 | 2.50 1.40-4.46 p = 0.002* |
| Total cholesterol: | p = 0.287 | ||||||||
| Median | 166.0 | 164.0 | 157.5 | 170.0 | 167.0 | 182.0 | p = 0.861 | p = 0.917 | |
| Interquartile range: 25th–75th percentile [mg/dl] | 137.0–215.0 | 142.0–209.0 | 132.0–204.0 | 143.0–203.0 | 145.0–196.0 | 143.0–217.0 | |||
| LDL-C: | p = 0.793 | ||||||||
| Median | 79.5 | 86.0 | 80.0 | 79.5 | 91.0 | 91.0 | p = 0.333 | p = 0.946 | |
| Interquartile range: 25th–75th percentile [mg/dl] | 64.5–119.5 | 61.0–106.0 | 59.0–110.0 | 54.0–103.5 | 55.0–116.0 | 47.0–113.0 | |||
| TG: | p = 0.829 | ||||||||
| Median | 136.5 | 120.0 | 135.0 | 105.5 | 111.0 | 133.0 | p = 0.152 | p = 0.644 | |
| Interquartile range: 25th–75th percentile [mg/dl] | 84.0–170.0 | 90.0–163.0 | 90.5–172.0 | 85.0–157.5 | 82.0–174.0 | 72.0–178.0 | |||
| HDL-C: | p = 0.579 | ||||||||
| Median | 53.0 | 51.0 | 55.5 | 59.0 | 51.8 | 52.0 | p = 0.050 | p = 0.465 | |
| Interquartile range: 25th–75th percentile [mg/dl] | 46.0–63.0 | 43.0–61.0 | 45.5–65.5 | 48.0–74.2 | 43.5–76.0 | 47.0–71.2 | |||
| Non-HDL-C: | p = 0.427 | ||||||||
| Median | 107.0 | 113.0 | 103.0 | 111.0 | 110.8 | 126.0 | p = 0.482 | p = 0.563 | |
| Interquartile range: 25th–75th percentile [mg/dl] | 86.0–157.0 | 81.0–148.0 | 80.5–146.0 | 85.5–131.6 | 86.0–137.2 | 89.0–154.0 | |||
OR – odds ratio, 95% CI – 95% confidence interval, p – statistical significance level, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, LDL-C – low-density lipoprotein cholesterol, TG – triglycerides, HDL-C – high-density lipoprotein cholesterol, non-HDL-C – atherogenic lipoprotein cholesterol,
Table II
PON1 alleles for the Q192R polymorphism and parameters of the lipid profile
| Components of the lipid profile | Patients after PCI or qualified for CABG (the study group), n = 280 | Patients with no significant lesions in coronary arteries (the control group), n = 284 | Statistical significance of differences between the groups | |||
|---|---|---|---|---|---|---|
| Q | R | Q | R | Q | R | |
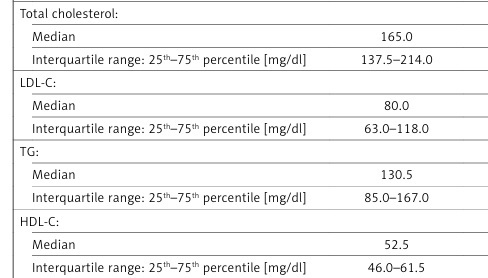
| n (%) OR 95% CI | 152 (54.3%) | 128 (45.7%) | 207 (72.9%) | 77 (27.1%) | 0.44 0.31–0.63 p = 0.000004* | 2.26 1.59–3.22 p = 0.000004* |
| Total cholesterol: | p = 0.986 | p = 0.128 | ||||
| Median | 165.0 | 161.5 | 170.0 | 175.0 | ||
| Interquartile range: 25th–75th percentile [mg/dl] | 137.5–214.0 | 137.0–204.0 | 143.0–203.0 | 143.0–206.0 | ||
| LDL-C: | p = 0.205 | p = 0.851 | ||||
| Median | 80.0 | 80.0 | 80.0 | 91.0 | ||
| Interquartile range: 25th–75th percentile [mg/dl] | 63.0–118.0 | 61.0–108.0 | 55.0–104.0 | 49.0–115.0 | ||
| TG: | p = 0.050 | p = 0.723 | ||||
| Median | 130.5 | 130.0 | 106.0 | 119.0 | ||
| Interquartile range: 25th–75th percentile [mg/dl] | 85.0–167.0 | 93.0–167.0 | 85.0–163.0 | 74.0–177.0 | ||
| HDL-C: | p = 0.003* | p = 0.281 | ||||
| Median | 52.5 | 53.0 | 59.0 | 52.0 | ||
| Interquartile range: 25th–75th percentile [mg/dl] | 46.0–61.5 | 45.0–65.0 | 47.0–74.5 | 45.0–73.0 | ||
| Non-HDL-C: | p = 0.237 | p = 0.561 | ||||
| Median | 108.0 | 103.5 | 110.8 | 115.5 | ||
| Interquartile range: 25th–75th percentile [mg/dl] | 86.0–150.0 | 81.0–148.0 | 86.0–132.0 | 88.2–145.0 | ||
OR – odds ratio, 95% CI – 95% confidence interval, p – statistical significance level, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, LDL-C – low-density lipoprotein cholesterol, TG – triglycerides, HDL-C – high-density lipoprotein cholesterol, non-HDL-C – atherogenic lipoprotein cholesterol,
Discussion
Despite intensive research, the biochemistry of PON1 remains poorly confirmed. The most important physiological role of PON1 is to delay the development of atherosclerosis by hydrolysing oxidised LDL (oxLDL) [11]. The enzymatic activity of a paraoxonase is influenced by genetic and environmental factors. So far, a number of polymorphisms within genes encoding paraoxonases have been identified. Among these, the Q192R polymorphism is considered to be the most significant in cardiovascular diseases: it can affect the active centre, and thus the qualities of the enzyme.
The 192QQ genotype was shown to play a protective role against the development of atheromas, which indicates that the RR genotype may be associated with a higher risk of coronary artery disease (CAD). The PON1 isozyme encoded by the R allele is less effective in inhibiting LDL oxidation as it poorly hydrolyses lipid peroxides [12]. An analysis of nine selected polymorphic sites within genes encoding paraoxonases showed that only the Q192R polymorphism affected the PON1 activity toward paraoxon. However, the authors indicated that the 192RR homozygotes presented the higher PON1 concentration and activity (p < 0.0001) compared to carriers of the QR and QQ genotypes [13].
The present study evaluated the influence of the Q192R PON1 polymorphism on the severity of atherosclerosis in coronary vessels in the Polish population. The 192RR genotype was present more frequently in the study group compared to the control group (31.4% vs. 15.5%; p = 0.002). Carriers of this genotype were at a 2.5-fold higher risk of atherosclerosis resulting in PCI/CABG (OR = 2.50, 95% CI: 1.40–4.46; Table I). Likewise, the 192R allele was associated with more than a twofold greater risk (OR = 2.26, 95% CI: 1.59–3.22; p = 0.000004; Table II). The QQ genotype was present in 40.0% of patients in the study group compared to 61.3% in the control group (p = 0.0004; Table I). The difference between the two groups in terms of the 192QR genotype frequency was not statistically significant.
In the study group, it was shown that HDL-cholesterol concentrations were significantly lower than in the control group (median: 53.0 mg/dl vs. 58.0 mg/dl; p = 0.023; data not shown in the tables). A similar relationship was observed in carriers of the 192Q allele (median: 52.5 mg/dl in the study group vs. 59.0 mg/dl in the control group; p = 0.003; Table II). No other significant differences concerning any other lipid profile parameters were found on both the genotype and allele level.
The are several limitations of the research including the total number of the participants (n = 282), data from a single centre in a country with a high CVD risk (Poland) and the lack of information on smoking status of the patients. Most patients were treated with statins, which may constitute a major confounder in this study.
In a similar study based on two groups of patients with CAD from an Iranian population, viz. those with stenoses of > 50% vs. those with stenoses of < 30%, the authors found that presence of the 192R allele to be associated with a higher risk of CAD [14]. Another study linked the RR genotype to higher mortality and increased frequency of cardiovascular events, including myocardial infarction and stroke. The frequency of these incidents was observed to be much lower in individuals with higher PON1 activity [15, 16]. Ochoa-Martinez et al. found the Q192R polymorphism to act as a genetic cardiovascular risk factor in a population of Mexican women [17].
Another group of researchers examined serum PON1 activity and associated genetic factors in 3668 patients without acute coronary syndromes (mean age ± SD: 63 ±11 yrs.) who had undergone elective coronary angiography. The study participants were prospectively observed for major adverse cardiac events (MACE) over a period of 3 years. It was found that the 192RR and 155MM PON1 genotypes strongly affected the serum PON1 activity but were not related to the 3-year risk of MACE [11].
In conclusion, the presence of the 192RR genotype and 192R allele was shown to be related to at least a two-fold increased risk of atherosclerosis requiring percutaneous coronary intervention or coronary artery bypass graft in the Polish population.