Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Patients with AF are at increased risk of thromboembolism and stroke [1]. More than 95% of thrombi are found in the left atrial appendage (LAA) [2]. During AF, contractility in the LAA is low, which decreases flow velocities and raises the risk of thrombus formation and stroke. Oral anticoagulation (OAC) is the preferred therapy for stroke prevention for patients with a CHA2DS2-VASc score ≥ 2 [3]. Atrial fibrillation is common in patients with chronic kidney disease (CKD), and 1-year mortality after stroke in patients with CKD is approximately 30% [4]. Additionally, patients with CKD have a high risk of bleeding complications under OAC [5]. In patients with severe chronic kidney disease OACs are contraindicated [6]. Novel anticoagulants such as rivaroxaban, dabigatran and apixaban have recently been demonstrated to exhibit non-inferior or superior efficacy to warfarin in clinical trials [7–9] and are indicated for use in non-valvular atrial fibrillation based on recent American College of Cardiology (ACC) and European Society of Cardiology (ESC) guidelines [10, 11]. However, major bleeding risk of OACs remain high and higher costs of OACs lead to poorer cost effectiveness [12]. Recently, LAA closure (LAAC) has been shown not to be inferior to OAC for stroke prevention, according to the PROTECT-AF and PREVAIL clinical trials [13–15]. Left atrial appendage closure thus plays an important role as an alternative to OAC for stroke prevention in patients with AF, reducing the bleeding risk [16–18], and could be a potential alternative to OAC in patients with AF and CDK. However, patients with CKD are well known to be at high risk for procedural complications and to have a worse outcome after transcatheter interventions [19–23].
The aim of our study was to examine the procedural safety and efficacy of LAAC in patients with AF and CKD.
Material and methods
The objective of the study was to evaluate the safety and efficacy of the percutaneous LAAC procedure in patients with CKD. Ninety-seven patients with AF and an indication for LAAC were included in this observational single-center study in the Department of Cardiology and Vascular Medicine at the West-German Heart and Vascular Center Essen between 01/2011 and 01/2017. The study was performed in accordance with the Declaration of Helsinki. The institutional Ethics Committee of the University of Duisburg-Essen approved the study protocol (16-7080-BO). All subjects provided informed consent.
Patients with at least one of the following conditions were eligible: bleeding complications while using OACs, bleeding history leading to markedly elevated risk of recurrence with OAC use, HAS-BLED score ≥ 3, difficulty managing the dose of warfarin to maintain a stable INR level, or OAC refusal.
The procedure was performed by well-trained and experienced operators under fluoroscopy and transesophageal echocardiography (TOE) guidance using dedicated Amplatzer devices (first- and second-generation), as previously described in detail. Left atrial appendage closure was performed with the patient under conscious sedation [24]. Intravenous heparin was administered as a bolus dose to achieve an activated clotting time (ACT) of > 250 s in all patients. Transesophageal echocardiography and an LAAC angiogram were used to determine the optimal device size. Based on instructions for use (IFU), all devices met the 5 signs of device stability (lobe compression, lobe perpendicular to LAA wall, disc of lobe with concave shape, disc separation from lobe, midpoint of lobe distal to left circumflex artery) prior to device release and were successfully implanted.
The post-implant drug regimen was discontinuation of OAC followed by dual-antiplatelet therapy with aspirin 100 mg and clopidogrel 75 mg daily for 3 to 6 months, following a loading dose of clopidogrel of 600 mg [15]. All patients were followed up with TOE after 6 months. Clinical follow-up to monitor major adverse events was done through outpatient or telephone assessments at 6 months. A long-term follow-up by telephone was carried out to monitor bleeding, stroke and mortality between 12 and 36 months after intervention.
Patient and procedural characteristics and follow-up results were collected in a dedicated database (Table I). In-hospital complications were reported for both groups including bleeding according to the ISTH definitions of bleeding in non-surgical patients [25], device embolization, need for surgery or cardiopulmonary resuscitation, pericardial effusion, and pericardial tamponade. Vessel complication was defined as access site vascular injury requiring surgical repair, access site-related bleeding requiring transfusion, or access site-related nerve injury that is permanent or requires surgery. Stroke was defined as an irreversible neurological deficit, as classified by the treating neurologist, on the basis of supporting information, including brain images and neurological evaluation.
Table I
Baseline characteristics of patients undergoing left atrial appendage closure grouped according to the presence of chronic kidney disease
Due to the number of patients included in this study we stratified patients according to CKD stage as normal to mild (KDOQI stage I–II) and moderate to severe (KDOQI stage III–V) CKD. The primary endpoint was safety of the procedure and complications within the 6-month post-procedure follow-up in patients with mild to moderate as compared to severe CKD. A 36-month long-term follow-up by telephone was carried out to control for bleeding complications, stroke and mortality.
Statistical analysis
The data were expressed with mean ± standard deviation (SD) and compared using the t-test or Mann-Whitney U test. Normal distribution was checked using the Kolmogorov-Smirnov test. Data analysis was performed with SPSS Statistics 22 (IBM for Windows). A p-value < 0.05 was considered statistically significant.
Results
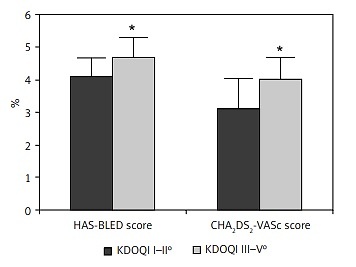
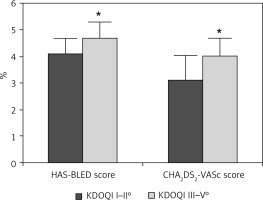
Ninety-seven patients (mean age of 73.9 ±8.5 years) with AF and contraindications for OAC or complications under OAC (i.e. gastrointestinal bleeding, intracranial bleed, poorly adjustable INR value) underwent LAAC with the Amplatzer Cardiac Plug and the Amplatzer Amulet Occluder. The baseline characteristics are listed in Table I. There was no significant difference in age (73.1 ±8.4 vs. 75.8 ±7.7 years), gender, body mass index (BMI) (27.6 ±4.7 vs. 28.6 ±5.4 kg/m2), coronary artery disease (60.4 vs. 69.3%) or hypertension (100%). Patients with severe CKD (n = 49) had significantly higher CHA2DS2-VASc and HAS-BLED scores and were at higher thromboembolic and bleeding risk (CHA2DS2-VASc: 4.08 ±0.79, HAS-BLED: 4.76 ±0.69) due to minimally higher age and more PAD than patients with mild to moderate CKD (n = 48, CHA2DS2-VASc: 3.69 ±1.1, HAS-BLED: 4.06 ±0.66; p < 0.001 for both). Calculated STS score and EuroSCORE II predicted higher thromboembolic, bleeding, and periprocedural risk in patients with severe CKD (Figures 1 and 2).
Figure 1
Periprocedural risk was elevated in patients with chronic kidney disease stage III–V. *p < 0.05
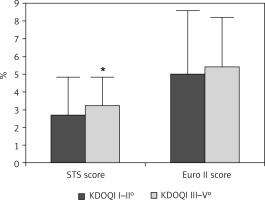
Figure 2
Patients with chronic kidney disease stage III–V° were at higher thromboembolic and bleeding risk. *p < 0.05
The primary endpoints were safety and successful device implantation, including complications during follow-up. Successful device implantation into the LAA was achieved in 97% of cases.
The rate of successful device implantation was 98% in the CKD I–II group and 96% in the CKD III–V group. None of the patients needed surgery, but 1 patient in each group developed serious pericardial effusion. None of the patients experienced periprocedural device embolization or vascular complications. During the 6-month and long-term follow-up between 12 months and 36 months after intervention, none of the patients in either group had bleeding complications or stroke (Tables II and III).
Table II
Periprocedural results of patients undergoing left atrial appendage closure grouped according to chronic kidney disease
Table III
Post-procedural follow-up between 12 months and 36 months after procedure
| Variable | KDOQI I–II (n = 48) | KDOQI III–V (n = 47) | P-value |
|---|---|---|---|
| Stroke, n (%) | 0 | 0 | |
| Bleeding complication, n (%) | 0 | 0 | |
| 36-month mortality, n (%) | 4 (8.3) | 5 (10.6) | 0.512 |
During the long-term follow-up 4 patients died in the KDOQI I–II group vs. 5 patients in the KDOQI III–V group (8.3% vs. 10.6%, p = 0.512) (Table III).
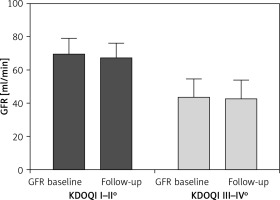
Secondary endpoints were the effects of contrast medium used (138.1 ±75.5 ml vs. 141.6 ±84.2 ml, p = 0.347) and subsequent changes in renal function. During the 6-month follow-up, renal function was unchanged in both groups (Figure 3).
Figure 3
Change in glomerular filtration rate (eGFR) in patients with mild to moderate and severe kidney disease
One patient in the KDOQI I–II group and 3 patients in the KDOQI III–V group had residual interatrial shunt after transseptal puncture (2.1% vs. 6.3%, p = 0.442), and 2 patients from the KDOQI I–II group and 1 patient from the KDOQI III–V group had leakage next to the device (4.2% vs. 2.1%, p = 574) (Tables II and IV).
Table IV
Adverse events during follow-up
Discussion
Left atrial appendage closure plays an important role as an alternative to OAC for stroke prevention in patients with AF who experienced complications under OAC. Patients with CKD have a high risk for cardiovascular morbidity and mortality and worsened outcomes after cardiovascular surgery and intervention.
Additionally, they are at high risk for complications such as bleeding, infections, and thromboembolism [26–28]. We investigated whether elevated periprocedural bleeding risk and stroke risk (STS score, EuroSCORE II, CHA2DS2-VASc score and HAS-BLED score) in patients with moderate to severe CKD (KDOQI stage III–V) results in equally successful intervention rates and low complication rates as compared to patients with normal or mild CKD (KDOQI stage I–II).
In our study population, the follow-up after 6 months and between 12 and 36 months after intervention showed similar results and a low general complication rate in both patient groups. Of the 95 patients who received LAAC no patient suffered from stroke or peripheral vascular complications. TOE showed similar intermediate results in terms of leakage (4.2 vs. 2.1%, p = 0.574) in patients with normal to mild (KDOQI stage I–II) CKD and moderate to severe (KDOQI stage III–V) CKD. It is known that patients with CKD undergoing invasive cardiology procedures have a higher risk of complications [22, 23].
In our study, patients with CKD had complex clinical conditions that may have led to bleeding, including pacemaker implantation, wounds, cancer and previous operations; however, compared to patients without CKD, the risk of major bleeding did not increase. Baseline hemoglobin was similar in both groups (12.6 ±3.1 vs. 11.9 ±2.9, p = 0.319) and did not change significantly in the follow-up. This may be because LAAC avoids long-term OAC therapy, which increases the bleeding risk.
The number of peri- and post-procedural complications during short-term and long-term follow-up in our study are surprisingly low. There was only one major complication, i.e. pericardial effusion or pericardial tamponade, in each group. No minor adverse events were reported. No patient needed cardiovascular surgery. Mortality during long-term follow-up was equal in both groups.
This is in accordance with other studies investigating the LAAC and chronic renal function. Kefer et al. [29] presented in their study the procedural safety and efficacy of LAAC with the ACP device. Stroke reduction was similarly high in patients with CKD and patients with normal renal function with no impact of the CKD stages on the periprocedural major adverse events. This is confirmed by Xue et al. [30], whose study using the Watchman Device documented no differences in complications in the CKD vs. the non-CKD group. Moreover, the observed bleeding rate reduction was significantly higher in the CKD group compared to the non-CKD group.
Left atrial appendage closure is not the first choice of therapy in patients with non-valvular AF, but studies have proven the non-inferiority of LAAC as compared to warfarin. Our study has shown that LAAC is safe, even in patients with advanced renal disease, despite increased procedural risk.
At present however, very few nephrologists are aware of and confident with LAA occlusion, a non-pharmacological alternative to oral anticoagulation, for their patients with CKD and AF. For this reason, these patients are rarely discussed with interventional cardiologists or with electrophysiologists for clinical evaluation. On the other hand, cardiologists are often not keen to perform invasive procedures in a fragile population such as renal disease patients, fearing major complications.
The present study has several limitations that should be acknowledged. This was a nonrandomized, retrospective, observational study, which included only 97 patients who underwent LAAC with the Amplatzer device. The follow-up with TOE and laboratory values was restricted to 6 months. Post-procedural long-term follow-up included a range from 12 months to 36 months. Long-term follow-up was restricted to bleeding complications and stroke. Only general mortality between 12 months and 36 months after the procedure could be determined.
In conclusion, in spite of a higher thromboembolic and bleeding risk in patients with severe CKD, LAAC is an equally safe and a feasible option for stroke prevention in patients irrespective of kidney disease stage.