Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
UROLOGY / EXPERIMENTAL RESEARCH
PLK1 downregulation by RO3280 prevents cell proliferation, migration, and invasion via the Wnt/β-catenin pathway in prostate cancer
1
Department of Urology, Liaoyang City Central Hospital, Liaoyang, China
2
Department of Urology, First Hospital of China Medical University, Shenyang, China
Submission date: 2021-04-01
Final revision date: 2021-08-10
Acceptance date: 2021-08-22
Online publication date: 2021-08-26
Corresponding author
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Higher expression levels of serine/threonine-protein kinase 1 (PLK1) are significantly associated with tumorigenesis and poor clinical prognoses. Consequently, PLK1 is considered a latent target in cancer treatment. We aimed to determine the cytotoxic effects of RO3280 on prostate cancer cells.
Material and methods:
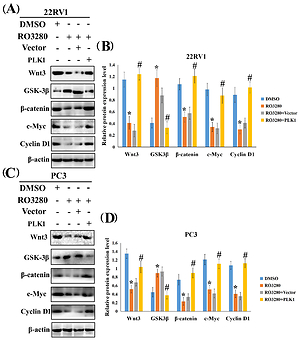
PLK1 expression was investigated using real-time PCR and western blotting in prostate cancer tissues and paired normal tissues. Real-time cell analysis, Cell Counting Kit-8 assays, and 5-ethynyl-2'-deoxyuridine cell proliferation assays were applied for the examination of cell proliferation ability. Wound healing assays and transwell assays were used to assess the migratory and invasive abilities of the prostate cancer cell lines with or without RO3280 treatment. Moreover, the target genes and pathways were detected by transcriptomics RNA sequencing in the cells cultured in RO3280 and through a series of bioinformatic analyses. Finally, the Wnt/β-catenin pathway was screened out and verified by western blotting.
Results:
We observed the mRNA and protein overexpression of PLK1 in the prostate cancer cells and tissues. The inhibition of PLK1 by RO3280 significantly reduced the migratory, invasive, and proliferative properties of the RO3280-treated cancer cell lines compared with their controls. RO3280 mediated the inactivation of the Wnt/β-catenin pathway, and reduced the rates of cell proliferation, migration, and invasion in prostate cancer cells.
Conclusions:
This study’s findings are significant owing to the identification of the specific anticancer mechanism of RO3280, which may have therapeutic effects. This trial provides clarity on the feasibility of the use of RO3280 as a cancer therapeutic agent for prostate cancer.
Higher expression levels of serine/threonine-protein kinase 1 (PLK1) are significantly associated with tumorigenesis and poor clinical prognoses. Consequently, PLK1 is considered a latent target in cancer treatment. We aimed to determine the cytotoxic effects of RO3280 on prostate cancer cells.
Material and methods:
PLK1 expression was investigated using real-time PCR and western blotting in prostate cancer tissues and paired normal tissues. Real-time cell analysis, Cell Counting Kit-8 assays, and 5-ethynyl-2'-deoxyuridine cell proliferation assays were applied for the examination of cell proliferation ability. Wound healing assays and transwell assays were used to assess the migratory and invasive abilities of the prostate cancer cell lines with or without RO3280 treatment. Moreover, the target genes and pathways were detected by transcriptomics RNA sequencing in the cells cultured in RO3280 and through a series of bioinformatic analyses. Finally, the Wnt/β-catenin pathway was screened out and verified by western blotting.
Results:
We observed the mRNA and protein overexpression of PLK1 in the prostate cancer cells and tissues. The inhibition of PLK1 by RO3280 significantly reduced the migratory, invasive, and proliferative properties of the RO3280-treated cancer cell lines compared with their controls. RO3280 mediated the inactivation of the Wnt/β-catenin pathway, and reduced the rates of cell proliferation, migration, and invasion in prostate cancer cells.
Conclusions:
This study’s findings are significant owing to the identification of the specific anticancer mechanism of RO3280, which may have therapeutic effects. This trial provides clarity on the feasibility of the use of RO3280 as a cancer therapeutic agent for prostate cancer.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.