Introduction
Cancer is one of the most significant global public health issues [1]. According to the Turkish Statistical Institute (TUIK), cancer was the second leading cause of mortality in Turkey in 2022, following viral and parasitic disorders. Moreover, 7.2% of malignancies in Turkey are recorded in women under the age of 54, according to data from TUIK [2]. Oncologic screening, diagnosis, and advancements in treatment have reduced mortality rates and improved the prognosis of cancer patients. Young survivors of these cancers, however, experience fertility issues as a result of chemotherapy [3].
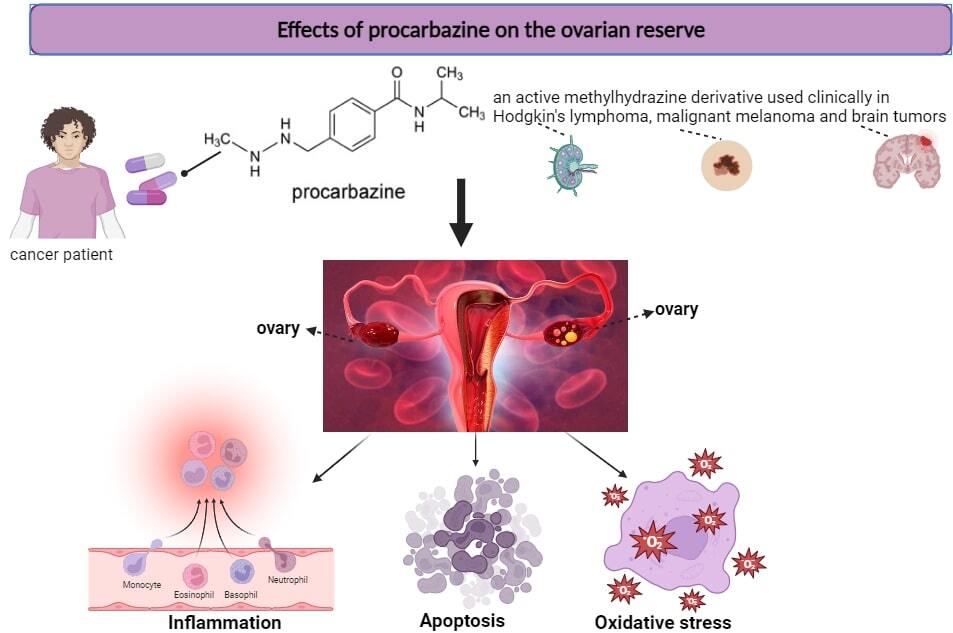
Indicative of a woman’s reproductive capacity, ovarian reserve represents the quantity and quality of follicles in the ovary at different stages of development. As an alkylating chemotherapeutic agent, procarbazine is recognized for its detrimental effects on ovarian reserve. Examining the drug’s mechanisms of action is crucial for minimizing ovarian damage and optimizing treatment protocols [4].
For this purpose, we first examined articles published in Google Scholar and PubMed until November 20, 2023. The titles of studies that included the terms “procarbazine”, “ovarian reserve”, or “chemotherapeutic” were assessed. The potential mechanisms of procarbazine-induced ovarian damage – inflammation, apoptosis, and oxidative stress – are exhaustively described in this review.
Procarbazine
Procarbazine, which is also referred to as p-toluamide, benzamide, PCB, PCZ, N-methyl-hydrazine, Natulan, and Matulane, is an orally active methylhydrazine derivative with a molecular weight of 257.76 and a molecular formula of C12H19N3O-HCl. It is clinically utilized in combination regimens to treat brain tumors, malignant melanoma, and Hodgkin’s lymphoma [5].
Chemotherapeutic agents frequently impact the ovary via diverse mechanisms, and recognizing which facets of ovarian function are impacted by these agents is essential. Oocytes and granulosa cells are vulnerable to injury caused by chemotherapy. Apoptosis, inflammation, and oxidative stress are the three principal mechanisms by which procarbazine is hypothesized to affect the ovaries, according to previous research [6].
Apoptosis
Apoptosis has a crucial role in eliminating germ cells during the process of oogenesis and following ovulation. The process of oocyte apoptosis involves various substances, including cAMP, cGMP, and calcium. Mitochondrial and cell surface receptor pathways are also utilized in this process. Apoptotic processes occurring in oocytes lead to the reduction of ovarian reserves, which has a direct impact on the reproductive results of mammals [7].
Disruption of cellular communication between granulosa cells and oocytes prevents the passage of cAMP, cGMP, calcium, and nitric oxide into follicular oocytes. The decrease in these molecules leads to reactive oxygen species (ROS) production, i.e., an increase in oxidative damage. Increased oxidative stress destabilizes maturation-promoting factors in diplotene stage oocytes, arrests the meiosis cell cycle, and induces apoptosis [8].
Calcium is an important signaling molecule. It has been suggested that the increase in intracellular calcium levels may lead to apoptosis in oocytes by inducing ROS formation through mitochondrial membrane polarization [9].
Oocytes may also undergo apoptosis in response to a reduction in antiapoptotic factors, an increase of proapoptotic factors (BH3 proteins), or the release of cytochrome c. Among the aforementioned factors, Bax protein and caspase-3 activation play a significant role. Cytochrome c shows its effect by activating caspases in apoptotic pathways. The most critical consequence of activating caspase-3 is the induction of DNA fragmentation [10]. TUNEL or ELISA techniques can thus readily detect apoptosis occurring even in a single oocyte. Procarbazine leads to damage of follicular oocytes through the aforementioned processes [11]. The depletion of germ cells in the ovarian reserve may be caused by apoptosis-mediated processes [12].
Inflammation
Inflammation is an essential response of the immune system to tissue damage and infection. It is usually characterized by slight increases in pro-inflammatory cytokines such as interleukin (IL) 6 (IL-6), tumor necrosis factor (TNF-α), IL-1β, and IL-18 and activation of the NLRP3 inflammasome in immune cells such as macrophages. It has been reported that TNF-α and IL-1β levels are high in mouse ovaries of reproductive age and that IL-1β and TNF-α, IL-8 and IL-6 levels are also increased. Decreased levels of anti-inflammatory IL-10 are an inflammatory response. Procarbazine may also show its effect on the ovaries via these markers [13].
Concentrations of different inflammatory markers in follicular fluid affect oocyte maturation, follicular wall rupture, and fertilization. In rodents treated with alkylating agents such as procarbazine, the NLRP3 (NOD-like receptor-3) and NF-κB pathways are activated, and inflammatory reactions occur. This is directly associated with decreased ovarian reserves [14].
Inflammatory cytokines regulate the secretion of steroid hormones required for follicle growth, ovulation, and corpus luteum development. Recent studies have reported reduced ovarian reserves in inflammatory conditions such as Crohn’s disease and polymyositis. In addition, ovarian reserves and oocyte quality are adversely affected by inflammation due to metabolic diseases such as obesity [15].
Oxidative stress
Oxidative stress arises from a disparity between the oxidant and antioxidant mechanisms within the cell, wherein the levels of reactive oxygen species increase while the activity of the antioxidant system decreases. An essential impact of chemotherapeutics, including procarbazine, is the suppression of antioxidant system function, specifically glutathione peroxidase, catalase, and superoxide dismutase. This inhibition is marked by increased oxidative stress and the buildup of ROS within the cell. These drugs also substantially elevate the amounts of malondialdehyde, which is the most critical end product of lipid peroxidation [16].
Critical cellular components can be damaged by oxidative stress, resulting in chromosomal damage, mutant cell formation, and aberrant cell growth. This is associated with ROS production and, as a result of chemotherapeutic effects, reduces antioxidant capacity [17]. Depletion of antioxidant defense mechanisms exacerbates the detrimental effects of oxidative stress. The reproductive function, including oocyte maturation, steroidogenesis, fertilization, and embryonic development, is profoundly affected by this process. While the exact mechanism remains unknown, it is hypothesized that procarbazine and its derivatives increase ROS production and decrease antioxidant activity in a dose-dependent manner as a result of oxidative stress [18].
ROS are generated in the mitochondrion, an organelle whose redox state fluctuates in response to biochemical and physiological stimuli. When pharmaceuticals such as procarbazine are used, a redox imbalance results [19]. Prolonged exposure to reactive oxygen species may affect follicular atresia, oocyte senescence, ovarian reserves, and granulosa cell apoptosis [20].
Conclusions
Several potential protective agents and methods have emerged in recent years to counteract the detrimental effects of chemotherapeutic treatments on the ovary. Available options include cryopreservation of embryos, oocytes, and ovarian tissue [21]. In addition, the literature has also described the use of agents such as sphingosine-1-phosphate, tamoxifen, crocetin, mechanistic targets of rapamycin complex inhibitors, and tyrosine kinase inhibitors to counteract the harm produced by alkylating drugs such as procarbazine. These agents have been reported to improve ovarian follicular and vascular development by preventing damage-induced apoptotic follicle death [22]. The inclusion of some of these agents in clinical trials is promising. Nevertheless, it is imperative to demonstrate that these drugs not only save the ovary from harm but also do not impede the efficacy of chemotherapy treatment.
Reduced ovarian reserves and, as a result, an elevated risk of infertility are recognized side effects of chemotherapy for women. The mechanisms by which alkylating drugs, such as procarbazine, affect ovarian reserves and injury are being elucidated through ongoing research. Nevertheless, a significant constraint and shortcoming is the lack of experimental evidence regarding these mechanisms in the literature. Future recommendations for novel treatment regimens to preserve fertility in at-risk women could be aided by resolving this issue. Given that the impact of procarbazine on ovarian reserve is intrinsically linked to the patient’s age, treatment regimen type, and dosage, comprehending these mechanisms will provide valuable guidance for the development of novel therapeutic interventions.