Introduction
Problems related with cognitive functions, increasing with age, are presented in many studies. Going through the menopause in women is also associated with a clear decline in cognitive functioning [1, 2]. It is still difficult to state the causes for the deterioration of cognitive functioning in women during this period of life. Many studies suggest a dominant role of decline of oestrogen levels related with the depletion of the ovarian reserve [3–5]; however, according to population studies, oestrogen therapies do not bring the expected improvement in this aspect [6–10]. Therefore, it seems that the problem is more complex and requires more comprehensive observations and research.
During the period of menopause, many women suffer from the symptoms of climacteric syndrome. These symptoms are both somatic and psychological complaints. The most frequent and most troublesome problem for women during the menopausal transition are vasomotor symptoms manifested by hot flushes and sweats. These symptoms considerably deteriorate the quality of life of women. The researchers investigated, among others, the relationship between vasomotor symptoms and disorders of cognitive functions. It has been suggested that vasomotor symptoms, through sleep disorders, may exert a negative effect on cognitive functions [11]. Other studies confirmed a direct effect of the deterioration of cognitive functions and sleep disorders [12]. Insomnia, sleep problems, or decreased quality of sleep exist in 40–60% of women during this period of life [13, 14]. Untreated sleeplessness is related with many serious health consequences, such as hypertension and diabetes, as well as psychical problems, such as depression [15–17].
Intensification of sleep problems during and after menopause has been observed in many studies, including large population studies such as the Study of Women’s Health Across the Nation (SWAN), the Australian Longitudinal Study on Women’s Health, and the Seattle Midlife Women’s Health Study [18–21]. Nevertheless, in studies in which many factors that may be responsible for the prevalence of sleep problems during menopause were taken into consideration, such as vasomotor and depressive symptoms, no correlation was found between sleep disorders and the serum oestradiol concentration in the examined women [22].
The lack of simple relationships between the decline in the oestrogen concentrations, and sleep disorders and cognitive disorders during the menopausal transition leads to the search for more complex mechanisms that might explain this. A potential way to explain these complicated relationships and simultaneously create new therapeutic possibilities is the identification of the genetic markers related with changes in cognitive functions, as well as changes in the quality of sleep during menopause. It is also a challenge to investigate whether the possession of individual polymorphisms of these genes can modulate the effect of sleep quality on cognitive functions during the period of menopause.
One of the candidate genes that may be responsible for the problems considered is the oestrogen receptor a gene (ESR1). In the central nervous system (CNS), ESR1 occurs in the hypothalamus, olfactory lobe, amygdale, and hippocampus; therefore, in the regions related with memory, mood, and the rhythm of sleep and wakefulness [23].
It was found that a decrease in the number and expression of ESR1 within the hippocampus is associated with impairment of hippocampus-related cognitive functions [24]. It seems that this is a weakening of ESR1 function and not a decrease in the number of oestrogen receptors, and it may be related with a weaker response to oestrogen therapy in women at an older age. The degree of weakening of the function of receptors may depend on ESR1 polymorphisms. The degree of expression of individual ESR1 polymorphisms is related with the weakening of the function of ESR1 to a varying degree, which may lead to the necessity for the modification of the oestrogen dose in order to improve its effective action [25, 26].
The genes encoding ESR1 have many polymorphic variants (approximately 9000). Among the most important and most frequently examined variants are two polymorphisms of the SNP type (single nucleotide polymorphism) – Xba1 and PvuII. The Xba1 (A/G rs9340799) polymorphism is located in intron 1 of the ESR1 gene, 5’ end at 351 bp upstream of exon 2, and is called IVS1-351. This is caused by A to G transition. Approximately 50 bp away from the Xba1 polymorphism site is positioned the PvuII (T/C, rs2234693) polymorphism, known as IVS1-397T/C [27]. This is caused by T to C transition in intron 1, 397 bp before the 5 ‘end of exon 2. Despite the location of PvuII polymorphism in the intron, it plays a major role in the regulation of expression of ESR1 protein [28]. The hypothesis was stated that oestrogen receptor a polymorphism modifies the correlation between sleep disorders and cognitive disorders.
The objectives of the study were: 1) analysis of the relationship between sleep disorders and cognitive disorders, and 2) analysis of the relationship between sleep disorders and cognitive disorders, according to the possessed oestrogen receptor a polymorphism in perimenopausal and postmenopausal women in non-manual employment.
Material and methods
Study group
The data were collected in the years 2016–2017. The study included 300 women aged 44–66 years, employed in various institutions as non-manual workers. The criteria of enrolment into the study were: proper age and non-manual employment. From the study, the following women were excluded: those who had moderately and strongly expressed menopausal symptoms assessed using the Kupperman Index, those who were ill with chronic diseases, dementia, those addicted to medicines, and women who applied hormone-replacement therapy. A brief MoCA test was performed in order to exclude the women who presented with features of dementia. Only women who obtained scores of at least 26 were included in the study.
Based on the STRAW criteria, the examined women were divided into two groups according to their reproductive status: perimenopausal period and postmenopausal period [29, 30]. The examined perimenopausal women were aged 44–56; mean age 49.5 ±3.2 years, while those during postmenopausal period were aged 46–66; mean age 56.4 ±3.4 years. Postmenopausal women were significantly less educated: 24.28% of them had university education, whereas among those who were perimenopausal – 35.33% (p < 0.001); the other women had secondary school education.
The women in the study had blood collected to measure oestradiol (E2) and follicle-stimulating hormone (FSH) concentrations. Blood samples were immediately taken to an accredited laboratory SYNEVO.
Informed consent for participation in the study was obtained from all the women.
The study was approved by the Ethics Committee of the Institute of Rural Medicine in Lublin, Poland.
Cognitive functions
Assessment of cognitive functions [31] was performed based on the cognitive functions diagnostic equipment CNS Vital Signs (Polish version), using the software by CNS Vital Signs (1829 E. Franklin St., Bldg 500, Chapel Hill, NC 27514, USA). The instrument, in the form of a battery of computer tests, was standardised, and was subjected to the full validation procedure. It has many cultural and language adaptations, including Polish, and the whole examination procedure was therefore performed in Polish. The report from the results, however, was printed in English. The presented cognitive functions were assessed in the following domains: Complex Memory, Verbal Memory, Visual Memory, Psychomotor Speed, Reaction Time, Complex Attention, Cognitive Flexibility, Processing Speed, Executive Function, Simple Attention, and Motor Speed.
The CNS Vital Signs clinical report provides standard scores of Neurocognitive Index (NCI) and 11 cognitive functions. Neurocognitive Index is automatically calculated based on five cognitive functions: memory, psychomotor speed, reaction time, attention, and cognitive flexibility. In addition, the clinical report classified NCI and every cognitive function into five groups: above average (standard score > 109), average (90–109), low average (80–89), low (70–79), very low (< 70).
Insomnia
Athens Insomnia Scale is an eight-item scale, which allows quantitative measurement of symptoms of insomnia according to the ICD-10 criteria [32]. Each of the eight questions consists of four possible options for the answer scored evaluated according to 0–3 scores, where 0 is the lack of a given symptom, while 3 is its considerable severity. The total result is within the range 0–24. After calculating the results obtained from the eight questions, the respondents were qualified into three groups: normal range, most probably not suffering from insomnia (≤ 5 scores); border of normal range, comply with the principles of sleep hygiene, and in the case of deterioration, consult a doctor (6–10 scores); and probably suffers from insomnia, should consult a doctor to supplement diagnostics and develop a strategy for therapy (> 10 scores).
DNA isolation
Genomic DNA isolation was derived from 0.2 ml of human blood by QIAamp DNA Blood Mini Kit (Qiagen, USA), as per the producer’s instructions. The amount and purity of the extracted DNA were measured using the NanoDrop spectrophotometer.
ESR1 polymorphisms
Polymorphisms of ESR1 were determined using the restriction fragment length polymorphism (RFLP-PCR) method. PCR reaction was performed in a total amount of 50 µl, containing: 1 U (1 µl) of DNA polymerase (Biotools), 1·PCR buffer (5 µl) containing 15 mM MgCl2 (Biotools), 2.5 µl 2 mM dNTPs (final concentration 0.1 mM) (Fermentas, Vilnius, Lithuania), 1 µl of 10 µM of each of the 2 primers, 34.5 µl nuclease-free water (Applied Biosystems Inc., USA), and 5 µl of genomic DNA. The reactions were performed in a C1000 Thermal Cycler (BioRad) and consisted of the initial denaturation (3 min at 95°C) and 30 cycles each of which included the proper denaturation (30 s at 95°C), primer annealing (50 s at 62°C), elongation (50 s at 72°C), and the final elongation (7 min at 72°C). Electrophoresis was performed in 2% agarose gel in standard conditions. The products of PCR (1372 bp) were digested overnight at 37°C using two separate restriction enzymes to determine the polymorphisms: PvuII (c.454-397 T > C) and XbaI (c.454-351 A>G). The products of restriction were electrophoresed in 2.5% agarose gel.
The alleles of the XbaI polymorphism were defined as A and G: heterozygote AG (fragments: 1372 bp, 936 bp, and 436 bp), homozygote GG (fragment: 1372 bp), and homozygote AA (fragments: 936 bp and 436 bp). The alleles of PvuII polymorphism were defined as T and C: heterozygote TC (fragments: 1372 bp, 982 bp, and 390 bp), homozygote TT (982 bp and 930 bp), and homozygote CC (1372 bp).
Statistical analysis
Statistical analysis was conducted with STATISTICA software (StatSoft, Poland). The absolute numbers (n) and percentages (%) were estimated for the categorical variables, and arithmetic mean (M) and standard deviation (SD) for continuous variables. The t-test for two means in independent samples was used to compare severity of insomnia and cognitive functions between perimenopausal and postmenopausal women, then between perimenopausal women and postmenopausal women with different genotypes of ESR1 polymorphism separately. F-test analysis of variance (with Bonferroni correction for multiple comparisons) was used to compare the severity of insomnia and cognitive functions between women with three different genotypes of ESR1 polymorphisms. Pearson’s correlation coefficient r was used to correlate the severity of insomnia and cognitive functions in the total group of women, in perimenopausal women, in postmenopausal women, in women with different genotypes of ESR1 polymorphisms separately, and in women in different reproductive periods and with different genotypes of ESR1 polymorphisms.
The significance level was assumed at 0.05.
Results
In the total group of examined women the following oestrogen receptor a polymorphisms were determined: XbaI AA in 134 women (44.67% of the total group of women in the study), AG in 127 (42.33%), GG in 39 (13.00%), PvuII TT in 87 (29.00%), TC in 147 (49.00%), and CC in 66 (22.00%). Both genotype distributions (XbaI and PvuII) were in line with Hardy-Weinberg equilibrium.
The mean severity of insomnia in the total group of women in the study was 6.70 ±4.66, which indicates the border of normal range on average; 141 (47.00%) had no insomnia, 105 (35.00%) were on the border of normal range, and 54 (18.00%) suffered from insomnia.
The NCI in the total group of women was a score of 92.6, on average, which indicates an average evaluation. The examined women obtained the best complex attention (101 scores, on average), while the worst reaction time (89 scores, on average). The other nine cognitive functions were in-between (mean values from 92 to 97 scores) (Table I).
Table I
Insomnia and cognitive functions in the total group of women as well as in perimenopausal and postmenopausal women
The severity of insomnia and cognitive functions were compared between two groups of women: perimenopausal women and postmenopausal women (Table I). The examined postmenopausal women obtained a significantly lower reaction time (87 scores, on average) than those of perimenopausal women (score of 91, on average). The other cognitive functions and severity of insomnia did not significantly differ between the examined peri- and postmenopausal women.
Serum oestradiol concentration positively correlated with processing speed only in postmenopausal women (r = 0.161; p = 0.044), while no correlation was observed with other cognitive functions, severity of insomnia, or ESR1 in peri- and postmenopausal women (p > 0.05).
Subsequently, the severity of insomnia and cognitive functions were compared between three groups of women with ESR1 XbaI genotypes: AA, AG, and GG, as well as between three groups of women with ESR1 PvuII genotypes: TT, TC, and CC. Severity of insomnia in the examined women was not significantly related with the possessed ESR1 genotypes, both XbaI (p = 0.612) and PvuII (p = 0.645), whereas the reaction time depended on the ESR1 genotypes possessed, both XbaI and PvuII. Women with genotype AA achieved significantly better reaction time (90.90 ±15.70 scores, on average) than women with GG (84.03 ±14.88 scores, on average) (p = 0.049), while reaction time did not significantly differ between women with AA and AG (87.88 ±18.69 scores, on average) (p = 0.456) or between women with AG and GG (p = 0.644).
Women with genotype TT obtained significantly better reaction time (91.86 ±14.65 scores, on average) than women with CC (85.24 ±18.37, on average) (p = 0.048), while reaction time did not significantly differ between women with TT and TC (88.44 ±17.51, on average) (p = 0.404) or between women with TC and CC (p = 0.613). The other cognitive functions in the total group of women were not significantly related with the genotypes ESR1 possessed – neither XbaI nor PvuII (p > 0.05).
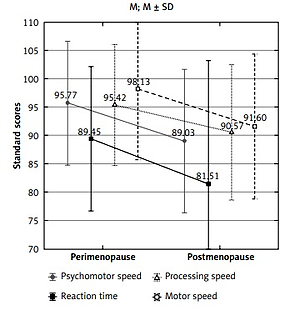
Next, the severity of insomnia and cognitive functions were compared between perimenopausal women and postmenopausal women with different genotypes of ESR1 polymorphism (Table II). In the group of women with genotype CC PvuII ESR1, the postmenopausal women had significantly lower psychomotor, motor, and processing speeds, as well as reaction time, in comparison with perimenopausal women (Figure 1). However, such a difference was not found in groups with TT and TC PvuII as well as with all genotypes of XbaI ESR1.
Table II
The p value for comparisons of insomnia and cognitive functions between perimenopausal women and postmenopausal women with different genotypes ESR1 polymorphism
Figure 1
Cognitive functions significantly differing between peri- (n = 31) and postmenopausal (n = 35) women with genotype CC PvuII ESR1
Subsequently, the correlations were investigated between severity of insomnia and cognitive functions in the total group, in perimenopausal women, and in postmenopausal women separately (Table III). Severity of insomnia negatively correlated with the following cognitive functions: complex and visual memories, and simple attention in the total group, i.e. the greater the severity of insomnia, the worse the complex and visual memories, and simple attention. Also, a negative correlation was confirmed between severity of insomnia and simple attention in perimenopausal women, i.e. the greater the severity of insomnia, the worse the simple attention. However, such a correlation was not found in postmenopausal women.
Table III
Correlations between severity of insomnia and cognitive functions in the total group, in perimenopausal women and in postmenopausal women
Next, correlations were investigated between severity of insomnia and cognitive functions in the groups of women with various genotypes of ESR1 polymorphisms (Table IV). A negative correlation was found between insomnia and simple attention in women with genotypes AG XbaI and TC PvuII ESR1, i.e. the greater the severity of insomnia, the worse the simple attention. Such a correlation was not found in other groups of women examined.
Table IV
Correlations between severity of insomnia and cognitive functions in groups of women with different genotypes ESR1 polymorphisms
Finally, the correlations were examined between severity of insomnia and cognitive functions in the groups of perimenopausal women with different genotypes of ESR1 polymorphism, as well as in the groups of postmenopausal women with these genotypes (Table V). Severity of insomnia negatively correlated with simple attention in perimenopausal women with genotypes AG XbaI and TC PvuII ESR1, with visual memory in women with TT Pvu II, and with reaction time in women with genotype AA XbaI post menopause.
Table V
Correlations between severity of insomnia and cognitive functions in groups of perimenopausal women with different genotypes of ESR1 polymorphisms, and in groups of postmenopausal women with these genotypes
Discussion
Our studies have shown that the cognitive functioning of the studied women was on an average level and that the severity of insomnia in the whole group of women was on the border of normal range. The severity of insomnia did not differ between perimenopausal women and postmenopausal women. However, the greater the severity of insomnia, the lower some cognitive functions in the group of studied women. The oestrogen receptor a polymorphisms did not correlate with the severity of insomnia. However, ESR1 polymorphisms correlated with the severity of some cognitive functions, depending on the reproductive status. In our study it was found that 18% of women suffered from insomnia. The results of studies by other researchers indicate that many peri- and postmenopausal women suffer from insomnia, poor quality of sleep, or both [33, 34]. The frequency of sleep disorders increased with age (from 39.7% in women aged 40–44, up to 45.2% in those aged 55–59 [35]. The greatest sleep disorders occurred in postmenopausal women [35].
In our study, postmenopausal women, compared to perimenopausal women, obtained significantly lower results only with respect to reaction time. Thus, it is not possible to confirm either a clear decline in the level of cognitive functions, or their relationship with the reproductive period. The results of studies concerning the relationship between menopause and the deterioration of cognitive functioning are inconclusive. In the study by Halbereich et al. the lack of a relationship was confirmed between age and the level of performance of the majority of tests in women before menopause. The occurrence of menopause clearly deteriorated the level of efficiency of some cognitive processes, which was interpreted by the researchers as an example of the effect of hormonal changes, especially the cessation of the protective role of oestradiol and progesterone [36, 37]. The study by Yaffe et al., conducted on a large population group, demonstrated that women with a low oestrogen level obtained significantly lower results in tests assessing complex attention, and immediate and delayed memory [38]. Deterioration of short-term memory, as well as episodic and verbal memory, are observations repeated in many studies concerning postmenopausal women [39]. Also, concentration and divisibility of attention deteriorate with age, as well as spatial orientation and processing speed [40, 41].
A study conducted in Poland in a group of 402 women (mean age: 56.5 ±3.5 years) showed the greatest deterioration of cognitive flexibility, processing speed, and executive function, whereas the lowest – of verbal and visual memories. The NCI was very low in 17.66% of the examined women, low in 19.9%, below average in 15.92%, average in 45%, and above average in 1.49% [42].
However, in the relevant literature there are reports indicating the lack of a relationship between menopause and the level of performance of tasks engaging cognitive processes [43].
Our own study confirmed that cognitive functioning may be related with sleep disorders. The greater the severity of insomnia, the worse the complex and visual memories, as well as simple attention, in the women examined. Very similar results were obtained by other researchers, who observed changes concerning attention and episodic memory in persons with insomnia [44]. A meta-analysis previously performed by this team of researchers demonstrated that persons suffering from insomnia showed impairment of efficiency in several cognitive functions, including working memory, episodic memory, and selected aspects of executive functioning [45].
Nevertheless, the results of some studies do not confirm the correlation between insomnia and worse cognitive functioning [46, 47].
The relationships between cognitive functioning and sleep disorders at menopausal age are not clearly confirmed by scientific studies; therefore, the question arises whether there are any additional factors, for example genetic, which may explain these ambiguities.
In own study, in women with genotypes AG XbaI and TC PvuII ESR1, a negative correlation was observed between severity of insomnia and simple attention. The severity of insomnia negatively correlated with reaction time in postmenopausal women with genotype AA XbaI, while with visual memory in those with PvuII TT. In turn, in perimenopausal women with genotypes AG XbaI and TC PvuII ESR1, severity of insomnia negatively correlated with simple attention. This might suggest that individual polymorphisms XbaI and PvuII ESR1 intensify the negative effect of insomnia on selected cognitive functions, especially on simple attention.
The studies conducted to-date demonstrate that persons with genotype XbaI GG remain stable or show improvement with respect to cognitive functioning [48], while those with genotype XbaI AA are at an increased risk of the development of AD [49, 50].
However, no studies have been found concerning the relationship between insomnia and cognitive functioning of women at perimenopausal age according to oestrogen receptor α polymorphism. This part of our own study seems to be innovatory and worthy of further comprehensive investigation.
The strengths of our study are the standardised research tools used to assess sleep disorders, and especially computer tests to assess cognitive functions impairment, which gives the opportunity to compare our results with those of other authors. The studied group was homogenous and relatively large in relation to the cost of genetic tests.
The limitations of our project resulted from the high costs of genetic studies, which limited the number of respondents and the type of polymorphisms analysed. It would certainly be reasonable to conduct a prospective study – at several time points in the perimenopausal period – regarding the problem being analysed.
In conclusion, the more severe the insomnia, the worse the complex memory, visual memory, and simple attention found in the total group of the examined women. In the group of women at perimenopausal age, the more severe the insomnia, the worse the simple attention.
Simple attention correlated negatively with severity of insomnia in women with genotypes AG XbaI and TC PvuII ESR1.
During the perimenopausal period in carriers of genotypes AG XbaI and TC PvuII ESR1 the more severe the insomnia the worse the simple attention. During the postmenopausal period, the severity of insomnia negatively correlated with visual memory in carriers of PvuII TT, and with reaction time in carriers of XbaI AA.
The results obtained indicate the need for further studies in the area of modulation of the effect of insomnia on cognitive functions by genetic polymorphisms in peri- and postmenopausal women. This may be important for prophylaxis and treatment of cognitive disorders during this period of women’s lives.