Lung cancer was the most prevalent cause of cancer-related deaths in 2020 in the European Union [1]. Such a high mortality rate keeps the scientific community focused on this disease, both for prevention and effective treatment. The second factor contributing to the global burden of lung cancer is obesity. Individual meta-analyses confirmed that obesity is an independent risk factor worsening both the morbidity and severity of lung cancer [2, 3]. On the other hand, obese patients respond much better to immunotherapy based on immune checkpoint inhibitors, which is another example of the obesity paradox [4]. According to the presented thesis, the relationship between obesity and lung cancer immunology seems unclear.
Programmed death ligand-1 (PD-1; also known as CD279) is a glycoprotein membrane receptor, the basic role of which is inhibiting the immune response targeted against autoantigens. The PD-1 pathway achieves it by two mechanisms: by lowering recruitment of regulatory T-cells (Treg) and by inducing apoptosis of valid T lymphocytes. Physiologically, PD-1 is expressed in various cell subpopulations, but the main ones are mature CD4+ and CD8+ lymphocytes and B lymphocytes. PD-1 expression is strongly regulated by many factors, different for each cell subpopulation, including cytokines, hormones and antigen-induced pathways [5, 6].
PD-1L is the main PD-1 ligand, whose mRNA expression is present among all organism cells, but is significantly higher among antigen-presenting cells (APC) – dendritic cells, macrophages, B lymphocytes, and activated lymphocytes T [6].
Due to the fundamental role of the PD-1/PD-1L axis in immuno-oncology, the authors decided to analyze the expression of PD-1L in obese lung cancer patients in comparison with people of normal weight. The initial hypothesis was that a better response to immunotherapy in obese patients is associated with higher expression of PD-1L in lung cancer cells.
Methods. The authors conducted a retrospective study in which they collected clinical data of 67 patients with lung cancer diagnosed in 2018–2022. The data collected concerned height, weight, and expression of PD-1L (which is routinely examined when assessing eligibility for immunotherapy) since the diagnosis of lung cancer. The authors measured body mass index (BMI) and assigned the patients to 4 groups: underweight (BMI < 18 kg/m2), normal weight (18 < BMI < 25 kg/m2), overweight (25 < BMI < 30 kg/m2), and obesity (BMI > 30 kg/m2). The statistical analysis was performed using Statistica software.
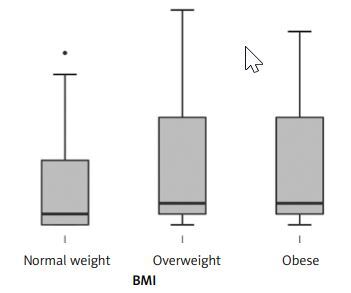
Results. In the studied population 12 (18.2%) people were obese, 33 (50.0%) were overweight and 21 (31.8%) had normal weight. The number of SCC was 29 (43.9%) and the number of AC was 37 (56.1%). Table I presents the number of cases divided into sex and BMI groups. Figure 1 presents the PD-1L expression in BMI groups. The analysis (Shapiro-Wilk test showing no normal distribution, and Kruskal-Wallis test) showed that there were no statistically significant differences in the PD-1L expression between BMI groups (p = 0.45). Moreover, there was no direct correlation between BMI and PD-1L expression (r = 0.25, p = 0.16; Figure 2). In the cancers histological subgroup analysis, there were no statistically significant relationships between BMI and PD-1L expression for both SCC (p = 0.39) and AC (p = 0.38).
Figure 1
PD-1L expression in BMI groups (normal weight (18 < BMI < 25 kg/m2), overweight (25 < BMI < 30 kg/m2) and obesity (BMI > 30 kg/m2))
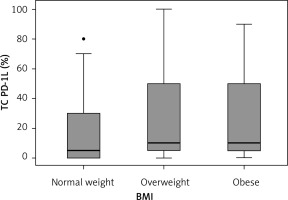
Figure 2
No significant correlation between BMI and PD-1L expression, using Spearmen correlation due to no standard distribution (r = 0.25, p = 0.16)
Table I
Number of cases (N) divided by histological subtype, sex and BMI interpretation
| Parameter | Women | Men | |
|---|---|---|---|
| SCC | NW | 3 | 6 |
| OW | 3 | 12 | |
| OB | 2 | 3 | |
| Total | 8 | 21 | |
| AC | NW | 8 | 4 |
| OW | 9 | 9 | |
| OB | 4 | 4 | |
| Total | 21 | 17 |
Then, the decision was made to conduct the PD-1L expression analysis separately for each gender. For women, using the ANOVA test (p = 0.050) with post-hoc analysis, a statistically significant difference in expression was found between women with overweight and women with normal weight (mean PD-1L expression for NW = 11.546, for OW = 39.750; p = 0.040), but not for obese women compared to normal weight women (p = 0.652). Due to the small number of participants in the retrospective study, female patients with squamous cell lung cancer and adenocarcinoma were collectively included in this analysis. For men, a similar approach did not yield any statistically significant differences (pANOVA = 0.717).
The χ2 test analysis did not show any correlation between BMI interpretation and the prevalence of specific histological types of lung cancer (pwomen = 0.932; pmen = 0.757).
Discussion. The binding of PD-1 to its ligands is a crucial mechanism for tumor cells to escape from the immune system. The higher expression of PD-1L on the surface of tumor cells allowed the patient’s lymphocytes to become anergic. Accordingly, PD-1/PD-1L axis-targeted immunotherapy is one of the most promising cancer treatments [7, 8]. Obesity is an independent risk factor for lung cancer development and is associated with increased tumor growth and aggressiveness. On the other hand, obesity is associated with improved response to cancer immunotherapy [4]. This result leads to the hypothesis that obese lung cancer patients should have a higher level of PD-1L expression compared to normal-weight patients. However, no statistically significant differences were found between BMI groups. On the one hand, the study presented in this article should be performed again, but including a larger group of patients, to be more reliable. But on the other hand, this may suggest that considering the PD-1/PD-1L axis in the context of the impact of obesity on lung cancer may be pointless. In this situation, other immunological mechanisms should be found and investigated (described below). The only significant difference was in PD-1L expression in overweight women compared to normal weight ones – but no corresponding difference was found comparing obese women to both overweight and normal weight ones – therefore the authors consider this significance a coincidence. The authors did not find any publication based on similar methods concerning the activity of the PD-1/PD-1L axis in obese lung cancer patients – therefore, they should be performed in order to confirm or refute the results presented in this paper.
Regarding other anatomical neoplasm sites, the impact of obesity on the immuno-oncology of the tumor microenvironment (particularly the PD-1/PD-1L axis) remains unclear. Studies on colon cancer (the pathogenesis of which is closely related to obesity) indicate the hypertrophy of adipose tissue as a negative factor in the activity of lymphocytes targeting cancer cells [9]. The aforementioned effect of obesity on the response to immune checkpoint inhibitor-based therapy has not been detected in melanoma [10].
The body mass index (BMI) as a diagnostic tool for obesity currently receives a mixed reception in the scientific literature. On the one hand, its limitations are emphasized, such as its restricted applicability in the pediatric population [11, 12] or its limited accuracy in patients with extensive muscle tissue [13–15]. Modern research highlights complementary applications of indicators such as waist circumference, hip circumference, or waist-to-hip ratio (WHR) [16]. These indicators allow both the diagnosis of obesity and the determination of its type (abdominal or gynoid). However, it is worth noting that these indicators show a high degree of correlation with BMI in general population studies [17], which is why their function in current obesity guidelines is usually complementary to the dominant BMI [18, 19].
On the other hand, BMI has numerous advantages, starting with its high availability and widespread use among medical personnel [20]. The ease of obtaining the measurement allows for broad application of the BMI indicator, from diagnosing obesity to scientific research, including randomized meta-analyses [19]. For instance, some studies on oncology identify obesity as an independent risk factor for various cancer types, including lung cancer [21–23].
The lack of strong evidence for our hypothesis suggests that other immune-oncological mechanisms must take part in bilateral interactions between obesity and lung cancer. Recent studies have indicated a significant role of the IL-17-Th1/Th17 pathway in the development of autoimmune diseases [24], chronic lung diseases [25] and – particularly – lung cancer [26]. The plausible extension of immunotherapy targeting this pathway from rheumatoid and allergic diseases to lung cancer also is currently being investigated [27]. The interactions between microbiota (mainly gut microbiota, but also less known lung microbiota) and the human immune system are also suspected to play a key role in lung cancer development [28], especially because a few studies link gut microbiota disturbances with the response to immune therapy in lung cancer patients [29].
From the hormonal perspective, the immunomodulatory properties of vitamin D [30] and adipokines [31, 32] are also being urgently researched in connection to lung carcinogenesis. The proper supplementation of vitamin D may reduce both the prevalence and mortality of lung cancer [33, 34].
In conclusion, according to the collected data, the expression of PD-1L does not seem to be a factor linking obesity with the pathogenesis of lung cancer. No significant difference was detected when comparing obese and overweight patients with normal weight ones.