Introduction
Diabetes is among the world’s most common chronic diseases. It is believed that globally, nearly 1 in 10 adults have diabetes, with nearly half a billion total patients [1, 2]. The burden of diabetes and its complications on patients, their caretakers, and healthcare systems is high [3]. Acute diabetic complications, such as severe hypoglycaemia or hyperglycaemia, may be life-threatening, whereas chronic complications can lead to substantial disability due to heart failure after myocardial infarction, loss of independence following a stroke, the need for dialysis due to kidney failure, leg amputation due to diabetic foot syndrome, or blindness due to retinopathy [4, 5].
Treatment achieving good glycaemic control can substantially reduce the risk of both acute and chronic diabetic complications [6, 7]. Currently, glycated haemoglobin (HbA1c) is the most commonly used indicator of long-term glycaemic control. However, HbA1c is an imperfect marker because it is a blood glucose (BG) level averaged over 3 months, and does not give information regarding the patient’s target fasting or postprandial BG levels [7, 8]. Similarly, HbA1c cannot reveal the frequency of hypoglycaemia or hyperglycaemia episodes, or information about glycaemic variability [9]. Self-monitoring of blood glucose (SMBG) with glucometers complements HbA1c measurements by providing the patient’s daily glucose levels, but gives only a limited number of glucose values per day, and is inconvenient and socially uncomfortable, which results in poor compliance and suboptimal glycaemic control [10, 11].
This review describes novel blood glucose (BG) monitoring methods, i.e., intermittently scanned continuous glucose monitoring (isCGM) and real-time continuous glucose monitoring (rtCGM), which can substantially improve short-term and long-term glycaemic control and reduce the disease burden on patients and their caretakers. Both these systems are increasingly being used worldwide, so it is important that specialists in diabetology, as well as family physicians, have practical knowledge about them.
Novel methods of glucose monitoring in diabetes care
IsCGM and rtCGM are new methods of monitoring BG in patients with type 1 or type 2 diabetes. In contrast to standard glucometers, isCGM and rtCGM devices do not require blood samples and, therefore, eliminate the need for multiple finger-sticks per day. This makes them not only more convenient but also a more hygienic method of glucose control. Instead, these devices use glucose sensors commonly placed percutaneously by patients themselves or, rarely, subcutaneously by medical professionals. In both methods, BG is measured continuously and non-invasively from interstitial fluid, and measurements can be read at any time [12]. In isCGM, BG readings are obtained on demand by scanning the sensor with a handheld reader or smartphone, whereas rtCGM provides constant measurements displayed on a device. Although isCGM readings are obtained on demand, the device stores 8 h of glycaemic data that are downloaded to the reader each time the sensor is scanned (Figure 1) [13]. Thus, full-day glycaemic readings are obtained by a minimum of three evenly distributed scans per day, allowing for retrospective analysis of glycaemic control. Many rtCGM devices need calibrating twice daily, requiring conventional glucose measurement with a glucometer. In contrast, the isCGM device (FreeStyle Libre) is factory calibrated, eliminating the need for finger-sticks. Table I compares the characteristics of rtCGM and isCGM with conventional glucometers.
Table I
Comparison of blood glucose measurement methods*
Figure 1
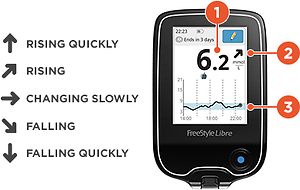
Smartphone app (FreeStyle LibreLink App) view. The device displays current blood glucose levels (1), a trend arrow (2, see interpretation on the left), and an 8-hour glycemia profile (3)
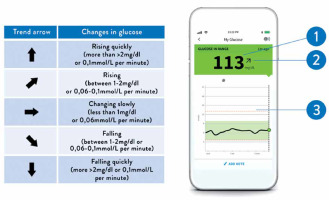
The convenience of the novel methods of BG monitoring improves compliance and, therefore, treatment outcomes. Data from a Scottish study show that patients with diabetes rarely check their BG, against medical recommendations [14]. In contrast, large studies show that patients with type 1 or type 2 diabetes using isCGM check their glucose much more frequently: 8–16 times per day [15–17]. In patients with both major types of diabetes, frequent BG monitoring is associated with better glucose control, including lower HbA1c values [18, 19]. Similar findings were observed in studies in which the use of CGM devices was associated with improved glycaemic control and reduced risk of both hypoglycaemia and hyperglycaemia compared to conventional SMBG monitoring [15–17]. Within the group of patients who used isCGM, glycaemic control was better among those who scanned their sensor more frequently [15, 20]. In addition to more frequent measurements, isCGM and rtCGM provide patients with information about BG trends over short periods (e.g., 15 min). These trends are displayed as up arrows (↑), down arrows (↓), horizontal arrows (→), or angled arrows (↗, ↘), single or double, which prompt the patient to take action to avoid hyperglycaemia or hypoglycaemia (Figure 1) [21].
New parameters of glycaemic control enabled by continuous glucose monitoring
The novel methods of glucose monitoring have introduced new parameters of glycaemic control to diabetes care. HbA1c monitoring combined with SMBG is insufficient for adequate glycaemic control, particularly in patients on intensive insulin therapy. Even patients with low HbA1c values have an increased risk of microvascular and macrovascular diabetic complications [22], possibly because HbA1c is an averaged measure, which could be the same for patients with BG ranges of 70–140 mg/dl or 40–210 mg/dl. HbA1c is unable to provide information about the time spent in the target BG range, hypoglycaemia, or hyperglycaemia. Data showing full-day glycaemic coverage from isCGM or rtCGM can be used to calculate new parameters of glycaemic control such as time in range (TIR), time below range (TBR), and time above range (TAR), which are becoming new treatment targets [23]. Devices for isCGM or rtCGM provide estimates of glycaemic variability (e.g., coefficient of variation); high glycaemic variability is a risk factor for diabetic complications and should be taken into account in diabetes management [24]. Additional parameters include an estimated value of HbA1c. Table II explains these new parameters of glycaemic control in more detail.
Table II
Recommended standardised CGM metrics for clinical care [23]
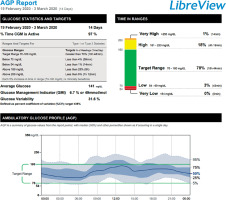
Reporting of the new parameters of glycaemic control is being standardised. Currently, a one-page Ambulatory Glucose Profile (AGP) is recommended (Figure 2), and is becoming part of routine diabetes care [25–27]. Treatment standards based on the parameters derived from CGM are in development [28].
Benefits of continuous glucose monitoring
The immediate, real-time benefits of CGM include the prevention and rapid detection of hypoglycaemia or hyperglycaemia due to more frequent measurements, device alarms, and trend arrows that prompt patients to prevent hypoglycaemia or hyperglycaemia by responding appropriately (e.g., eating a meal, increasing insulin dose). Many studies among patients with type 1 or type 2 diabetes show that CGM significantly reduces the time spent in hypoglycaemia or hyperglycaemia [16, 17, 23, 29–34]. Less time spent in dysglycaemia also decreases glycaemic variability [15]. Of note, a higher scanning frequency is associated with better glycaemic indices in patients using isCGM [15, 35].
A long-term, retrospective analysis of data from isCGM or rtCGM devices can help in the following areas:
Providing a general assessment of glycaemic control and identification of areas to be improved: reduced frequency and duration of hypoglycaemia/hyperglycaemia episodes; reduced BG variability and associated microvascular and macrovascular complications; reduced HbA1c levels [23, 29–34, 36].
Identification of events responsible for episodes of hyperglycaemia or hypoglycaemia (e.g., which meals are associated with the greatest increase in the glucose level, at what time of the day and night the BG increases or decreases).
Assessment of the impact of physical exercise, diet, stress, or other factors on BG.
CGM offers benefits for healthcare systems as well. The data recorded by these devices can easily be shared electronically with healthcare professionals, enabling tele-consultations. The CGM devices do not require strips for measuring BG. Preventing severe episodes of hypoglycaemia or hyperglycaemia can also reduce the incidence of hospitalisation of diabetes patients [37].
The isCGM and rtCGM systems provide new, clinically relevant information on glycaemic control and address the shortcomings of HbA1c monitoring alone. For example, an increased HbA1c value indicates that diabetes treatment should be modified, but it does not specify which areas need to be improved (e.g., hyperglycaemia, hypoglycaemia, glycaemic variability). In contrast, new parameters of glycaemic control included in an AGP report indicate which aspects of treatment should be changed [23].
Effects of novel glucose monitoring methods on patients’ everyday life
To maximize the benefits of isCGM and rtCGM, patients must be trained to use particular devices, i.e., placing percutaneous glucose sensors, using dedicated display devices and applications, and responding appropriately to BG readings and other information such as trend arrows.
Percutaneous sensors may be placed by patients themselves, which requires training. Subcutaneous sensors (Eversense) are placed by healthcare professionals in a relatively invasive procedure. Although rtCGM devices reduce the number of finger-sticks, most (all but Dexcom G6) still require a twice-daily calibration using a conventional glucometer. Conveniently, the isCGM device (FreeStyle Libre) is factory calibrated, eliminating the need for finger-sticks and the potential for errors related to faulty calibration.
An obvious advantage of CGM devices is the reduction or elimination of painful finger-sticks needed to measure BG with a glucometer. This is particularly important for children, who may not understand the concept of glycaemic control and are sensitive to pain. Parents of children with diabetes can use CGM to measure BG at night without waking their child, e.g., to rule out nocturnal hypoglycaemia, which can also improve the child’s sleep quality [38]. Checking CGM device readings is much more subtle than taking a finger-stick measurement with a glucometer; therefore patients feel more comfortable checking their BG in social situations.
CGM devices can increase both treatment compliance and disease awareness among patients with diabetes. Patients who use a CGM system check their BG much more frequently compared to conventional SMBG. Consequently, patients become more aware of their glycaemic control and are more likely to comply with their physicians’ recommendations. Owing to the availability of complete glycaemic coverage, patients can identify how particular activities, meals, or stressors in their daily lives affect their BG, and the additional information guides appropriate insulin dosing.
The ability to share glycaemic data electronically offers many advantages. It can improve patient-physician cooperation and give more control over BG to caretakers of patients with diabetes.
There are some specific populations, in addition to type 1 diabetes patients, that could particularly benefit from using CGM. These populations include children, pregnant women with diabetes and elderly patients. Generally, all patients on multiple daily injections or insulin pumps, including patients with type 2 diabetes and other specific types, reduce time spent in hypoglycaemia or hyperglycaemia, and thus have better glycaemic control. Patients with diabetes who use CGM devices report an improved quality of life and treatment satisfaction [17, 37, 39–41], reduced fear of hypoglycaemia, better work attendance [37, 42], fewer hospitalisations, and lower health-care costs [43]. As a result, there are many projections and simulations exploring the implementation of these devices in all patients with type 1 and type 2 diabetes [44–48].
Continuous glucose monitoring in telehealth
Telehealth interventions are an effective means of achieving glycaemic control in patients with diabetes [49]. CGM systems offer valuable glycaemic data that can easily be shared electronically between patients and healthcare professionals. Most CGM systems offer cloud-based platforms for sharing glycaemic data (LibreView, CareLink, Eversense DMS, Clarity). This enables a large proportion of consultations to be carried out remotely. Such tele-consultations are more convenient, less time-consuming and more cost-effective. The current pandemic of coronavirus-19 (COVID-19) has highlighted another benefit of telehealth for diabetes patients: a reduced risk of acquiring infectious disease in healthcare facilities, which are characterised by a high risk of viral transmission.
Conclusions
The novel methods to monitor BG continuously substantially improve care among patients with type 1 or type 2 diabetes. Data from CGM devices are used to facilitate better treatment compliance and outcomes and are improving routine diabetes management by providing new parameters for glycaemic control. Increased availability of CGM devices could be achieved by raising awareness among both patients with diabetes, and physicians of all clinical specialties. Subsidies should be considered for many patients worldwide, particularly because CGM systems can reduce some diabetes treatment costs by preventing hospitalisation and missed work due to diabetic complications. Data from clinical trials and observational studies showing improved glycaemic indices and quality of life should lead healthcare providers to consider wider CGM use in their patients.