Introduction
Gastroesophageal reflux disease (GERD) is characterized by its primary symptoms resulting from the reflux of contents into the esophagus and its specific complications, including reflux esophagitis, peptic strictures, and Barrett’s esophagus [1]. In recent years, the annual incidence of reflux esophagitis (RE) has been on the rise, attributed to improved living standards, changes in lifestyle, and dietary habits. RE has emerged as a significant public health concern in Asia [2]. RE can lead to the development of Barrett’s esophagus (BE) or esophageal adenocarcinoma (EAC), which can profoundly impact a patient’s quality of life and long-term prognosis [3]. BE serves as a precursor lesion for EAC and is a consequence of extended exposure to gastroduodenal refluxate [4–6]. Prevention of RE is a key approach to mitigate the risk of developing BE and EAC. Therefore, it is imperative to understand the causes of RE and implement preventive measures to reduce the risk of these conditions [7].
There exists a negative correlation between reflux esophagitis (RE) and Helicobacter pylori infection, whereas a positive correlation is observed with factors such as age, male gender, smoking, alcohol consumption, obesity, and hiatal hernia [8–10]. Furthermore, several studies have indicated that metabolic syndrome (MetS) is an independent risk factor for RE [11, 12]. MetS is defined as a cluster of metabolic abnormalities encompassing abdominal obesity, hypertension, hyperglycemia, low levels of high-density lipoprotein cholesterol (HDL-C), and elevated triglycerides (TG) [13, 14]. In a case-control study conducted in China, a high waist-hip ratio, hyperglycemia, hypertriglyceridemia, and MetS were all found to be associated with RE [15, 16]. Despite various studies showing that each Mets component is a risk factor for RE, these endocrine parameters have not been thoroughly assessed in predicting the severity and progression of RE.
Therefore, identifying metabolic parameters is essential for disease control and reducing RE. This study aimed to develop a nomogram for predicting the risk of RE based on routine metabolic parameters, to allow the early detection of high-risk groups, and to institute effective preventative or therapeutic measures to prevent and delay the complications of RE.
Material and methods
Study population
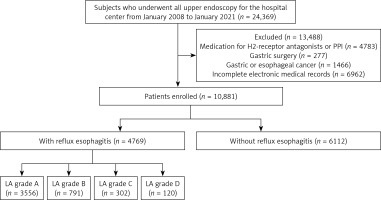
This single-center retrospective study was performed at the First Affiliated Hospital of Dalian Medical University, Dalian, China. A total of 24,369 subjects who received thorough medical evaluations, including physical examination, blood tests, and upper gastrointestinal endoscopy (GIF-H260, -HQ260; Olympus; Tokyo, Japan) between January 2008 and January 2021 were enrolled. The study excluded participants who had undergone previous surgery on the gastrointestinal tract (n = 227), were currently taking drugs such as H2-receptor antagonists or proton pump inhibitors (n = 4783), had been previously diagnosed with gastric or esophageal cancer at the time of gastroscopy (n = 1466), or had incomplete data (n = 6962). Finally, 10,881 participants were recruited for the analysis. They were categorized into two groups: 4769 individuals with reflux esophagitis and 6112 individuals without reflux esophagitis. Subsequently, individuals with reflux esophagitis according to the Los Angeles classification were further divided into four specific groups: LA-A with 3556 individuals, LA-B with 791 individuals, LA-C with 302 individuals, and LA-D with 120 individuals (Figure 1). This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics and Research Committee of the First Affiliated Hospital of Dalian Medical University (PJ-KS-KY-2020-04).
Data collection
Diagnostic methods for RE
The definition of RE was established through the outcomes of upper gastrointestinal endoscopy. All recruited individuals were categorized into two groups: those with RE and those without RE. Additionally, we employed the Los Angeles classification of esophagitis (LA) to determine the severity of RE [17]. A double endoscopy specialist independently confirmed the endoscopic findings for each subject.
Anthropometrics
Subjects were interviewed to collect data on their personal medical history, clinical characteristics, and self-reported race. Additionally, their body weight and height were measured and recorded. The assessment of personal medical history included information on diabetes mellitus, hypertension, surgery, and malignancy. All participants underwent anthropometric assessments while wearing lightweight undergarments, with measurements taken after fasting and voiding. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). For each subject, BMI was computed, categorizing individuals as obese if BMI > 30 kg/m2, non-obese if BMI ≤ 30 kg/m2, overweight if BMI was between 25 and 30 kg/m2, and normal weight if BMI was < 25 kg/m2 [18]. Blood pressure measurements were taken at the end of the physical examination with the participant in a seated position, with a minimum of 10 min of rest provided before the measurements.
Laboratory indicators
Blood specimens were obtained after an overnight fast of a minimum of 8 h. The Roche Cobas c701 automatic analyzer (Roche Diagnostics, Germany) was employed to quantify various biochemical parameters, including albumin (Alb), glucose (Glu), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), and uric acid (UA). Furthermore, laboratory indicators related to thyroid function, such as free triiodothyronine (fT3), free thyroxine (fT4), and thyroid-stimulating hormone (TSH), were analyzed using the Beckman Coulter UniCel DxI 800 Chemiluminescence immunoassay analyzer (Beckman Coulter, USA). All blood samples were processed within a 24-hour timeframe at the Medical Laboratory Center of the First Affiliated Hospital of Dalian Medical University.
Evaluation of H. pylori infection
H. pylori infection was defined as a positive outcome in either the 13C-urea breath test (UBT) or the rapid urease test. Specimens obtained through endoscopic biopsy were fixed with formalin and confirmed using Giemsa staining to establish the presence of H. pylori. In the case of the rapid urease test, a positive result was determined by a noticeable color change to pink or red after 24 h at room temperature.
The test procedure involved initially collecting a breath sample from the patient following a 4-hour fasting period. Subsequently, the patient ingested 100 mg of 13C-urea powder (UBiTkit; Otsuka Pharmaceutical, Tokyo, Japan) dissolved in 100 ml of water. After a 20-minute interval, a second breath sample was obtained, and a positive outcome was defined by a measurement exceeding the cutoff value of 2.5‰. The collected samples were then subjected to analysis using an isotope ratio mass spectrometer (UBiT-IR300; Otsuka Pharmaceutical).
Statistical analysis
We utilized SPSS (Version 26.0; SPSS Inc. (IBM, Armonk, NY, USA)) for the execution of statistical analyses. Image editing was carried out using GraphPad Prism 9.0. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were represented as numbers (N) with percentages (%). The comparison of quantitative and qualitative data between the two groups was conducted using the T-test and χ2 test. To assess the risk factors for RE, univariate and multivariate logistic regression analyses were employed, resulting in the calculation of odds ratios (OR) and 95% confidence intervals (CI) for each variable. In multivariate analysis, binary logistic regression analysis was applied to binary classification variables, such as survival status, while ordinal logistic regression analysis was utilized for ordered classification variables, such as the disease severity of RE.
For the development of a predictive model and a nomogram for RE, multivariable logistic regression analysis was employed. To evaluate the discrimination and calibration of the model, we utilized receiver operating characteristic (ROC), decision curve analysis (DCA), clinical impact curve (CIC), and calibration curve analyses. Data analysis was carried out using R version 3.3.2 (http://www.R-project.org,The R Foundation) and Free Statistics version 1.5. The threshold for statistical significance was set at p < 0.05 (two-sided).
Results
Characteristics of subjects enrolled in the present study
The study characteristics are shown in Table I. A total of 10,881 individuals underwent screening with esophagogastroduodenoscopy. After a review of the endoscopic findings by two experienced endoscopists, 4769 (43.8%) individuals were confirmed to have reflux esophagitis, including 3556 (74.6%) in LA-A, 791 (16.6%) in LA-B, 302 (6.3%) in LA-C, and 120 (2.5%) in LA-D. The mean age of subjects with RE was higher than that of subjects without RE (p < 0.001). There were more females than males with RE (M : F ratio of 2021 : 2748 vs. 2661 : 3451) in subjects without RE. However, there was no significant difference between the two groups in terms of sex and race. Metabolic parameters, including BMI, SBP, DBP, LDL-C, TG, TC, UA, fT3, fT4, and Glu, were significantly higher (p < 0.001), and HDL-C and Alb was significantly lower (p < 0.001) in subjects with RE compared to those without RE. In contrast, there were no significant differences in the indicators of ALT, AST, GGT, and TSH between the two groups. There were significant differences in the distributions of smoking, alcohol consumption, history of hypertension, and diabetes mellitus between the groups (p < 0.001). In addition, a significant difference in the distribution of H. pylori was also noted between the groups with and without RE (p < 0.001).
Table I
Demographic characteristics and clinical parameters of the study population
[i] BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, LDL-C – low-density lipoprotein-cholesterol, Glu – glucose, HDL-C – high-density lipoprotein-cholesterol, TG – triglycerides, TC – total cholesterol, ALT – alanine aminotransferase, AST – aspartate aminotransferase, GGT – γ-glutamyl transpeptidase, UA – uric acid, TSH – thyroid-stimulating hormone, fT3 – free triiodothyronine, fT4 – free thyroxine.
Univariate and multivariate analyses of factors associated with RE
Table II presents univariate and multivariate logistic regression analyses and predictors of RE. According to univariate logistic regression analysis, older age, alcohol consumption, smoking, hypertension, diabetes mellitus, H. pylori infection, TG, TC, HDL-C, LDL-C, higher BMI, higher SBP and DBP, hyperglycemia, UA, fF3, and fT4 were all positively related to RE. However, sex, race, GGT, TSH, ALT, and AST showed no significant differences between the groups with and without RE. A multivariate binary logistic regression analysis was then adopted to screen out the related factors for RE. The group under 40 years old was used as the reference group. Individuals aged 40–60 years (OR = 1.90; 95% CI: 1.52–2.39; p = 0.001) and more than 60 years (OR = 2.20; 95% CI: 1.78–2.75; p < 0.001) had a significantly higher risk of RE, with the association strengthening with age (p < 0.001). In addition, BMI showed an independent association with RE. The reference group for comparison was those with a BMI under 25 kg/cm2. The overweight group (BMI: 2–30 kg/cm2) demonstrated an increased risk of RE (OR = 1.31; 95% CI: 1.20–1.43; p = 0.001), while the highest BMI group (BMI > 30 kg/cm2) exhibited a higher risk of RE (OR = 1.46; 95% CI: 1.30–1.64; p = 0.015). Furthermore, multivariate logistic regression was performed to evaluate other potential factors related to RE. It showed that alcohol consumption (OR = 2.20; 95% CI: 1.88–2.57; p < 0.001), hypertension (OR = 1.53; 95% CI: 1.26–1.86; p < 0.001), diabetes mellitus (OR = 1.71; 95% CI: 1.35–2.17; p < 0.001), H. pylori positivity (OR = 3.81; 95% CI: 3.21–4.67; p < 0.001), SBP (OR = 1.02; 95% CI: 1.01–1.03; p < 0.001), DBP (OR = 1.27; 95% CI: 1.21–1.33; p < 0.001), Glu (OR = 1.08; 95% CI: 1.04–1.13; p < 0.001), LDL-C (OR = 1.27; 95% CI: 1.21–1.33; p < 0.001), HDL-C (OR = 0.70; 95% CI: 0.62–0.81; p < 0.001), TG (OR = 5.07; 95% CI: 4.29–6.00; p < 0.001), TC (OR = 1.27; 95% CI: 1.20–1.33; p < 0.001), Alb (OR = 0.88; 95% CI: 0.87–0.89; p < 0.001), UA (OR = 1.01; 95% CI: 1.01–1.02; p < 0.001), fT3 (OR = 1.09; 95% CI: 1.05–1.14; p < 0.001), and fT4 (OR = 1.11; 95% CI: 1.08–1.13; p < 0.001) were significantly and independently associated with RE.
Table II
Factors associated with reflux esophagitis using univariate and multivariate logistic regression
[i] See abbreviations under Table I.
Accuracy of the nomogram for predicting RE
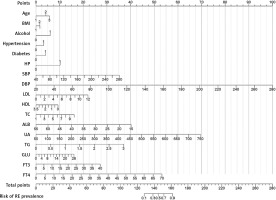
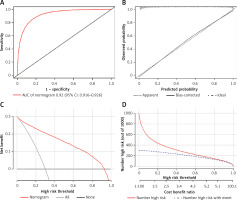
To display the prediction model vividly, a nomogram was constructed using the risk factors determined by the multivariate regression analysis (Figure 2). Then, we applied ROC to analyze the performance of the prediction model. The AUC of the nomogram for RE was 0.921 (95% CI: 0.916–0.926), with a specificity of 84.02%, a sensitivity of 84.86%, and an accuracy of 84.39% (Figure 3 A). As shown in Figure 3 B, the calibration curve of this model was relatively close to the ideal curve, which indicates that the predicted results were consistent with the actual findings. The decision curve indicates that when the threshold probability is between 30% and 90%, the application of this nomogram would add a net benefit when compared with either the RE or the No-RE strategies (Figure 3 C). The clinical impact similarly showed the strong clinical value of the nomogram (Figure 3 D).
Figure 3
The discrimination and calibration assessment of the model. A – ROC curve and AUC of the nomogram. B – Calibration curve of the nomogram predicting RE. C – Decision curve for the predictive nomogram. D – Clinical impact curve for the predictive nomogram
Each patient’s risk of RE was estimated by plotting the values of each variable on a graph. A vertical line was drawn from each value to the top points scale, which determined the number of points assigned based on the variable’s value. The points from all variables were then summed. This cumulative sum on the total points scale was located and projected vertically onto the bottom axis, resulting in a personalized risk assessment for RE.
Correlations between clinical and metabolic parameters associated with RE severity
The analyses conducted according to RE severity are shown in Table III. Statistical differences with risk factors were metabolic parameters and lifestyle factors. The following metabolic and lifestyle factors were significant and independent predictors of RE severity (p < 0.05): HDL-C, LDL-C, TC, TG, UA, fT3, fT4, alcohol consumption, smoking, hypertension, and diabetes mellitus.
Table III
Results of predictors of reflux esophagitis severity
[i] See abbreviations under Table I.
Effect of risk factors on RE severity
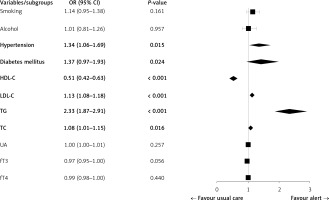
The ordinal logistic regression revealed that the risk factors predicted RE severity. The study identified several independent risk factors associated with the severity of RE. These included hypertension (OR = 1.34; 95% CI: 1.06–1.69; p = 0.015), diabetes mellitus (OR = 1.37; 95% CI: 0.97–1.93; p = 0.024), HDL-C (OR = 0.51; 95% CI: 0.42–0.63; p < 0.001), LDL-C (OR = 1.13; 95% CI: 1.08–1.18; p < 0.001), TG (OR = 2.33; 95% CI: 1.87–2.91; p < 0.001), and TC (OR = 1.08; 95% CI: 1.01–1.15; p = 0.016). However, smoking, alcohol consumption, UA, fT3, and fT4 were not found to be independent risk factors for RE severity (Figure 4).
Figure 4
Results of logistic regression analysis of the risk factors for reflux esophagitis severity
LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, TG – triglycerides, TC – total cholesterol, UA – uric acid, TSH – thyroid-stimulating hormone, fT3 – free triiodothyronine, fT4 – free thyroxine.
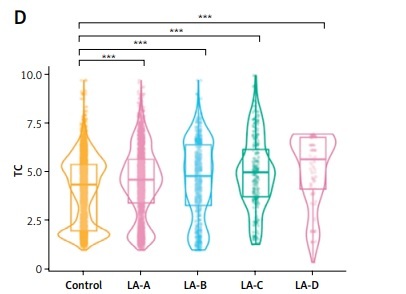
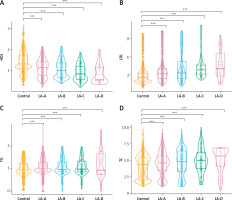
Relationship between HDL, LDL, TG, and TC in subjects without RE and those with RE of various severity
The above results indicate that HDL, LDL, TG, and TC are independent predictors of RE severity. Figure 5 shows significant correlations between HDL, LDL, TG, and TC levels and LA-A, LA-B, LA-C, and LA-D in patients with RE, compared to those without RE. HDL showed an overall lower level and a downward trend in LA-A to LA-D compared to those without RE (p < 0.001) (Figure 5 A). Meanwhile, LDL showed an overall higher level and an upward trend in LA-A to LA-D compared to those without RE (p < 0.001) (Figure 5 B). Furthermore, TG showed an overall higher level and an upward trend in LA-A to LA-D compared to those without RE (p < 0.001) (Figure 5 C). Moreover, TC showed an overall higher level and an upward trend in LA-A to LA-D compared to those without RE (p < 0.001) (Figure 5 D). These results show variation in the severity of RE based on the presence of lipid metabolism disorders.
Figure 5
Comparison of high-density lipoprotein cholesterol (HDL) (A), low-density lipoprotein cholesterol (LDL) (B), triglycerides (TG) (C) and total cholesterol (TC) (D) values between patients without reflux esophagitis and reflux esophagitis of various severity. P-values were determined using two-tailed t-test; ns, not significant; *p < 0.05; **p < 0.01; **p < 0.01; ****p < 0.001
Discussion
RE is a type of disease whose primary pathological mechanism is acid reflux [19]. The incidence of RE continues to increase worldwide. It has seriously increased the burden of global public health [20]. Therefore, the early recognition of RE is crucial. This study examined the presence of reflux esophagitis and the risk factors associated with its severity through a retrospective study. We found that routine metabolic parameters were associated with RE. Most importantly, the following factors were associated with RE: SBP, DBP, Glu, LDL-C, HDL-C, TG, TC, Alb, TSH, fT3, fT4, and UA. Particularly, based on the multivariate analysis, TG, TC, and LDL-C showed a strong positive association with severe RE, as defined by LA grades A–D. In contrast, HDL-C was inversely related to RE severity. Furthermore, we found that age, BMI, alcohol consumption, hypertension, diabetes mellitus, and H. pylori were significantly associated with an increased prevalence of RE. So, we utilized logistic regression to screen variables and selected 17 variables to construct a prediction nomogram model. This nomogram model enables direct prediction of RE prevalence and facilitates early identification of high-risk groups, thereby allowing timely implementation of preventive and treatment measures. Furthermore, we assessed the discrimination ability, calibration ability, and clinical practicability of the model. The results demonstrated that the prediction model performed well and exhibited clinical applicability.
Several reports have shown that obesity, age, and smoking are risk factors for RE [21, 22]. Chung et al. [23] determined that weight gain in overweight or obese groups may be more likely to support the development of RE than in the normal weight group. Therefore, BMI changes are a risk factor for new-onset RE. Our study results also demonstrated that obesity was a risk factor for RE. Obese individuals exhibit a higher incidence of RE compared to both overweight and normal-weight populations.
Age was associated with increased esophageal exposure to acid because of a progressive decrease in abdominal lower esophageal sphincter (LES) length and esophageal motility [24]. Furthermore, this study clarified that alcohol is a cause of RE. Several studies have shown that consumption of large amounts of alcohol can promote the regurgitation of acid into the esophagus, inducing RE, which is consistent with our conclusion [25, 26]. The relationship between RE and H. pylori is a topic of controversy. Some researchers have examined the endoscopic, esophageal 24-h impedance-pH monitoring of patients with H. pylori infection and GERD to better understand the relationship between the two, finding that H. pylori infection reduces stomach acidity and GERD severity and is one of the best predictors of RE risk [27]. However, H. pylori increases the acid sensitivity of the esophagus, which increases symptom development. It was revealed in the present study that RE increased in prevalence due to H. pylori infection, which is consistent with the above findings. There is uncertainty about the relationship between smoking and RE. Nam et al. [28] also suggested that smoking was not significantly associated with RE. In contrast, a study showed that smoking played a role in developing severe RE in the Japanese population [29]. This study showed that the prevalence of smoking in RE patients was higher than in controls and that it was not an independent risk factor for RE. Smoking decreases esophageal sphincter pressure, affects esophageal defense, and reduces esophageal clearance and saliva secretion, which impairs the ability of the esophagus to remove acid [30].
Several previous studies have found hypertension to be significantly associated with RE, which is consistent with our findings, though the mechanism underlying this association is uncertain [31, 32]. Calcium channel blockers are commonly administered as a treatment for hypertension. Calcium channel blockers have been shown to have the power to suppress the contractions of the esophageal muscle and ease the pressure on the esophageal sphincter. Several studies have found that calcium channel blockers are significant risk factors for RE [21]. The present study found that high SBP and high DBP were also independently correlated with RE and that hypertension was a risk factor for RE prevalence, even in severe RE. These results reflect that increased awareness of hypertension can contribute to the prevention and treatment of RE.
Studies have shown that variations in Glu levels can have an impact on RE [33, 34]. High Glu levels can increase stomach acid production, thereby worsening RE symptoms. However, low Glu levels have been observed to lower the tone of the lower esophageal sphincter (LES), a muscle that helps prevent acid reflux [35]. In our study, there were more patients with diabetes in the RE group compared to the no-RE group, and this difference was statistically significant. After conducting a multivariable analysis, high Glu levels were an independent risk factor for RE prevalence.
Hypertriglyceridemia and low HDL-C are both manifestations of dyslipidemia. Both conditions are risk factors for reflux esophagitis, which is supported by evidence from some previous studies. The study found that high-fat diets can lead to elevated triglyceride levels and other abnormal blood lipid levels, which can impair esophageal clearance and weaken the function of the lower esophageal sphincter, thereby contributing to the onset of RE [36]. In our study, TG, TC, LDL-C, and HDL-C were significantly associated with RE. In addition, lipid metabolism disorders were significantly related to RE severity. To change the condition, people with RE should be urged to adjust their lifestyles, such as losing weight, quitting smoking, and reducing alcohol consumption.
The significant independent predictors of RE included fT3, fT4, Alb, and UA in this study. There is currently no evidence to suggest a causal relationship between Alb and reflux esophagitis. Oh et al. [37] found that Alb was possibly associates with the nutrition of patients experiencing gastroesophageal reflux symptoms. We found a negative association between albumin levels and RE. In addition, in the present study, fT3 and fT4 were significantly higher in the RE group than in the non-RE group, and both were significant risk factors for RE. This result may be explained by gastrin release being influenced by the β-adrenergic system. Hypergastrinemia of thyrotoxicosis is mediated by hyperresponsiveness of the β-adrenergic receptors on the G-cell [38]. The increased secretion of gastrin stimulates gastric wall cells to produce a higher amount of gastric acid, thereby promoting the occurrence of RE [39]. Recent research revealed that serum uric acid may be considered as a predictor of metabolic disorders [40]. Kuwabara et al. [41] reported that elevated UA increased the risk of developing high LDL cholesterol, as well as hypertriglyceridemia. The mechanism is probably that first, visceral adipose tissue produces some adipocytokines that promote the development of insulin resistance, while hyperinsulinemia, in turn, reduces renal excitation of UA, leading to hyperuricemia [42]; second, decreased HDL and internal visceral fat accumulation can accelerate UA production, which in turn leads to more hepatic neutral fat synthesis, activating the UA synthesis pathway [43]; finally, the high deposition of sodium urate in the kidney causes hyperalgesia, leading to high HDL emission and a large accumulation of LDL [44]. These results revealed a relationship between UA and lipid metabolism disorders, which collectively lead to the occurrence of RE. In our study, UA was a risk factor for reflux esophagitis.
This study had several notable strengths. First, the study included a large sample and the diagnosis of RE was made by the trained gastroenterologist who performed endoscopy. Second, metabolic parameters used in the model are baseline characteristics that can be acquired easily. Third, our model showed good discrimination and was found to be accurate using the AUC and calibration. Despite its contributions, our study is not without its drawbacks. First, the present study was retrospective. Therefore, a multicenter randomized controlled clinical study with a larger sample size could be performed in the future to verify the clinical benefits. Second, although we adjusted for potential confounders in the multivariable analysis, there remained unmeasured residual confounding factors, such as hiatal hernia, dietary patterns, psychosocial stress, medication habits, and socioeconomic status, which may influence our risk estimates. Lastly, the study did not clarify the pathophysiological mechanism responsible for the close relationship between RE and metabolic disorders. This should be addressed using animal studies in the future.
In conclusion, metabolic parameters are associated with the symptoms of RE in patients. In particular, HDL-C, LDL-C, TG, and TC are associated with RE severity. These findings suggest the importance of identifying metabolic parameters in studies on the prevalence of RE. Among these parameters, blood lipid metabolism disorders have been found to exert the most significant influence on RE severity. Furthermore, the new RE prevalence model for patients with metabolic disorders constructed in this study had excellent identification and calibration ability, which was helpful for clinical practice.