Introduction
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disease causing symmetric polyarthritis (synovitis). Rheumatoid arthritis affects 0.5–1% of the world population and is most commonly seen between 30 and 50 years of age. Moreover, RA is more common in women than in men. On the other hand, RA is a heterogeneous disease that can be accompanied by extra-articular involvement such as cardiovascular, hematologic, pulmonary, vascular, etc. [1]. The limitation of basic life activities due to joint damage adversely affected the quality of life in RA patients [2]. The primary goal in the treatment of RA is to prevent joint damage by keeping synovitis under control. Methotrexate (MTX) is a disease-modifying antirheumatic drug (DMARD) commonly used in the treatment of RA, which is also known as an anchor drug. However, for patients with DMARD-resistant active RA, tumor necrosis factor α (TNF-α) inhibitors are an important treatment option [3]. The TNF-α inhibitors have been shown to be effective as monotherapy and combination treatment in randomized studies. Infliximab (INF) is the first monoclonal antibody to human TNF-α developed for RA treatment. INF binds with high affinity to both soluble and trans-membrane TNF and can decrease synovial inflammation, bone resorption, and cartilage degradation [4].
Neutrophils play a key role in the activation, regulation, and effector functions of innate and adaptive immune cells by releasing various cytokines and chemokines. Additionally, neutrophils have a high cytotoxic potential due to their role in the release of destructive enzymes and reactive oxygen species (ROS). When activated improperly, neutrophil extracellular traps (NETs), which are recruited to kill extracellular microorganisms, act as a source of autoantigens. Therefore, neutrophils play a major role in the pathogenesis of diseases such as infections and chronic inflammation in addition to autoimmune diseases [5]. Although there are numerous cells participating in the pathogenesis of RA, neutrophils play the central role among others. As a result, neutrophils from patients with RA often have an increased tendency to generate NETs containing citrullinated proteins that are commonly implicated in the pathophysiology of RA [6].
Oxidative stress (OS) is the impairment in the balance between oxidants and antioxidants in favor of oxidants. OS is known to have a role in the pathogenesis and progression of numerous inflammatory diseases including RA. Moreover, the role of OS in inflammation and joint injury has also been shown in RA patients and in animal studies. Lipid, protein, and DNA oxidation is greater in RA patients compared to healthy individuals, which results in the production of ROS, thereby leading to joint injury [7, 8]. Transient receptor potential melastatin 2 (TRPM2) is a Ca2+-permeable channel and is abundantly expressed in inflammatory cells including neutrophils. TRPM2 has been shown to act as a sensor for ROS and OS [9]. Increased OS leads to TRPM2 activation. Additionally, modulation of calcium ion entry through the TRPM2 channel has been shown to decrease apoptosis and OS markers [10]. The MTX and INF therapies are known to decrease OS markers in RA patients [11]. Meaningfully, it is likely that these two drugs, which are highly important for RA treatment, use the TRPM2 channel to exert their effects. The aim of our study was to investigate the effects of MTX and INF on apoptosis, oxidative stress and calcium ion entry through modulation of the TRPM2 channel in the neutrophils of patients with RA. In addition, in light of the findings, we also aimed to shed light on the effect of these two important drugs on the TRPM2 channel with regards to disease remission.
Material and methods
Patients and controls
The study was conducted at the BSN Health Analyses ARGE Ltd., Teknokent, Suleyman Demirel University (SDU) Campus, Isparta, Turkey. The patients enrolled in the study were selected from the patients admitted to SDU Medical School Rheumatology Department. The study was approved by the local ethics committee. The ethics committee approval number was 2017/144. All participants gave written consent, confirming their acceptance for giving blood through the vena brachialis and were informed about the experimental procedures. Demographic characteristics, clinical records, physical examination findings, and laboratory tests were noted for each patient.
The study included 10 patients with newly diagnosed RA and 10 age- and gender-matched control subjects. The diagnosis of RA was established based on the 2010 ACR/EULAR classification criteria for RA [12]. All the patients had active RA. Clinical Disease Activity Index (CDAI), Simple Disease Activity Index (SDAI), Disease Activity Score-28 (DAS-28), useful clinical composite scores that are used for evaluating disease activity in RA were administered to assess disease activity. The visual analog scale (VAS) was also used for assessing disease activity.
Inclusion criteria were as follows: patients who were aged between 18 and 60 years and fulfilled the 2010 ACR/EULAR classification criteria for RA. Exclusion criteria were as follows: concomitant diseases such as diabetes mellitus, hypertension, obesity, hyperlipidemia, depression, autoimmune diseases other than RA, active infection, tobacco or alcohol use, steroid or immunosuppressive treatment within the 4 weeks prior to blood sampling, malignancy or pregnancy, use of vitamin supplements, and previous exposure to biologics or DMARDS. Age- and gender-matched controls without any infection and no tobacco, alcohol or drug use were included in the study.
Serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), cyclic citrullinated peptide antibodies (anti-CCP), uric acid, alanine aminotransferase (ALT), hemogram, and venous blood fasting glucose level (FBG) were measured in each patient after 8-hour overnight fasting. Serum and neutrophil samples were separated from the blood samples. Neutrophil samples were used to measure intracellular Ca2+ concentration ([Ca2+]i) and were also used for the MTT, apoptosis, intracellular ROS production, mitochondrial depolarization and caspase 3 and 9 activation assays.
Groups
The neutrophils isolated from the patients and the controls were divided into four groups as follows:
Control group (n = 10): neutrophils obtained from the healthy subjects were not incubated by INF or MTX.
RA group (n = 10): no incubation was performed for the neutrophils obtained from the patients.
RA + INF (n = 10): neutrophils obtained from the patients were incubated with INF (0.1 mM) for 1 h [13].
RA + MTX group (n = 10): Neutrophils obtained from the patients were incubated with MTX (0.1 mM) for 1 h. The dose and the time of MTX incubation were determined using the cell viability (MTT) test.
In the Ca2+ signaling experiments, the effect of TRPM2 on Ca2+ entry was investigated in the neutrophils. To achieve this, the neutrophils in the four groups were further incubated with TRPM2 channel blocker, ACA (0.025 mM), for 10 min before fMLP stimulation. Both fMLP and ACA were purchased from Santa Cruz Inc, (Istanbul, Turkey) and their stock solutions were dissolved in DMSO. Before diluting in extracellular buffer with Ca2+ (1.2 mM), pH adjustment (7.4) was performed both for the agonists and antagonists.
Isolation of human neutrophils
Neutrophils were isolated from anti-coagulated peripheral blood of the patients and the healthy subjects as described previously [13–15]. Neutrophil isolation was carried out in sterile solutions containing phosphate-buffered saline (PBS) (GIBCO Invitrogen, Istanbul, Turkey), 6% hydroxyl ethyl starch solution in isotonic NaCl (Plasmasteril, Fresenius, Bad Homburg, Germany), and Ficoll-PaquePlus Solution (GE Healthcare Bio-Sciences, Uppsala, Sweden). Half of the neutrophils were used for the measurement of [Ca2+]i, MTT, apoptosis, intracellular ROS production, mitochondrial depolarization levels, and caspase 3 and 9 activation. The loading buffer contained HEPES (20 mM), NaCl (138 mM), KCl (6 mM), MgCl2 (1 mM), CaCl2 (1.2 mM), and glucose (5.5 mM) with a pH of 7.4. Remaining neutrophils were frozen in the buffer for measuring lipid peroxidation, reduced glutathione (GSH) level, and glutathione peroxidase (GSHPx) activity.
Cell viability (MTT) assay
Cell viability assays were performed by measuring mitochondrial reductase activity with MTT as described previously [10, 16]. The neutrophils were incubated in the MTT solution (0.5 mg/ml) for 15 min. The resulting formazan crystals were dissolved in dimethyl sulfoxide (DMSO; 200 μl/well). Optical density was measured with a spectrophotometer at 550 and 620 nm and presented as fold increase over the pretreatment level (experimental/control).
Measurement of [Ca2+]i in neutrophils of patients
The [Ca2+]i in the neutrophils of patients was measured as described previously [10, 17]. The neutrophils (1 × 106 neutrophil/ml) were loaded with 2 μM Fura-2/AM for 30 min in the dark at 37°C for 45 min. The fMLP was used to stimulate Ca2+ entry. Fluorescence was recorded by a spectrofluorometer (Carry Eclipse; Varian, Sydney, Australia) with excitation wavelengths of 340 and 380 nm at an emission of 505 nm. Changes in [Ca2+]i were monitored as the fluorescence ratio (Fura-2/AM 340/380 nm). Intracellular calibration for Ca2+ was performed as previously described. Ca2+ entry was estimated using the integral of the rise in [Ca2+]i for 170 s after fMLP stimulation. Ca2+ release was expressed in nanomoles by noting a reading every second [13, 14].
Neutrophils are known to be activated by increased [Ca2+]i [18]. Additionally, stimulation of neutrophils by bacterial fMLP is known to induce an increase in [Ca2+]i [19]. Nevertheless, recent reports have shown the modulator role of INF in [Ca2+]i through the inhibition of TRPM2 and voltage-gated calcium channels in patients with ankylosing spondylitis. In addition, the modulator role of MTX in oxidative stress in neutrophils stimulated by fMLP has also been reported [20].
Assay of apoptosis and caspase 3 and 9 activities
For the spectrophotometric analysis of apoptosis, a commercial kit was used and the analyses were performed according to the instructions provided by Biocolor Ltd. (Northern Ireland) and elsewhere [17]. In this APOPercentage dye-uptake assay, when the membranes of apoptotic cells loses their asymmetry, the APOPercentage dye is actively transported into the cells, staining apoptotic cells red, thus allowing detection of apoptosis by spectrophotometry.
The determinations of caspase 3 and 9 activities were based on a method previously reported by Kose and Naziroglu [21] with minor modifications. Cleavages of caspase 3 (ACDEVD-AMC) and 9 (AC-LEHD-AMC) substrates were measured with a microplate reader (Infinite pro200; TecanMännedorf, Switzerland) with an excitation wavelength of 360 nm at an emission of 460 nm. The resulting data were calculated as fluorescence units/mg protein and presented as the fold increase over the pretreatment level (experimental/control).
Intracellular ROS measurement
Intracellular ROS formation in the neutrophils was spectrofluorometrically estimated using a fluorescent probe, dihydrorhodamine 123 (DHR123), which is oxidized to a fluorescent dye, rhodamine (Rh123), by cellular oxidants, particularly by superoxide radicals [22]. The neutrophils (1 × 106 cells/ml) were first incubated in the presence of 10 μM DHR123 for 15 min in incomplete RPMI 1640 medium containing 10 mM HEPES buffer solution at 37°C. At the end of probe loading, the cells were washed with PBS. The kinetics of RH123 fluorescence intensity (excitation: 488 nm, emission: 530 nm) resulting from the oxidation of DHR123 were measured with a plate reader (Infinite pro200).
Measurement of mitochondrial membrane potential (ΔΨm)
JC1 accumulates in the mitochondria depending on the ΔΨm level and is present either as a monomer or a reversible J-aggregate. The JC1 monomer predominating in depolarized mitochondria emits green fluorescence at 530 nm, whereas the oligomer (J-aggregate) forming in mitochondria with negative potentials emits red fluorescence at 590 nm [23]. The green (excitation: 485 nm and emission: 535 nm) and red (excitation: 540 nm and emission: 590 nm) JC1 signals were measured in the cell line as described in a previous study [24]. Fluorescence changes were analyzed using a microplate reader (Infinite Pro200). The data were presented as the fold increase over the pretreatment level.
Lipid peroxidation analyses
Lipid peroxidation in the neutrophils was measured using malondialdehyde (MDA) reaction via the method proposed by Placer et al. [25]. The lipid peroxidation values in the neutrophils were expressed as micromoles per gram of protein. The protein content in the neutrophil samples was measured by the method described by Lowry et al. [26] with bovine serum albumin as the standard.
Reduced glutathione (GSH) and glutathione peroxidase (GSH-Px) assays
The GSH content of the neutrophil samples was measured spectrophotometrically (UV-1800; Shimadzu, Kyoto, Japan) at 412 nm according to the method proposed by Sedlak and Lindsay [27]. GSH-Px activity of the neutrophil samples was also measured spectrophotometrically at 37°C and 412 nm according to the method described by Lawrence and Burk [28].
Measurement of non-toxic MTX dosage
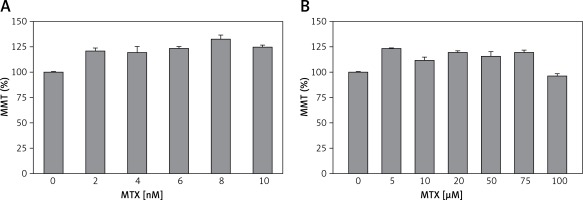
Literature reviews indicate that there is no study in the literature reporting on the dosage and incubation time of MTX in human neutrophils. However, previous studies indicate that in human dendritic cells [29] and intestinal mononuclear cells [30], therapeutic doses of MTX have been used between 0.5 and 5 nM. Therefore, in our study, the neutrophils obtained from the healthy controls were incubated with five different doses of MTX (2, 4, 6, 8 and 10 nM) for 1 h but no evaluation was performed for the effects of each dosage on the MTT levels (Figure 1 A). Previous studies also indicated that in human chondrocytes, MTX was used up to 100 μM. Therefore, we increased the dosage of MTX to determine its non-toxic dosage. The neutrophils obtained from the healthy controls were incubated with six different high doses of MTX (5, 10, 20, 50, 75 and 100 μM) for 1 h (Figure 1 B) and we found that the incubation of neutrophils for 1 h with 0.1 mM MTX was not toxic for the cells (Figure 1).
Figure 1
Effects of different doses of MTX on cell viability (MTT) test in the neutrophils of controls (mean ± SD and n = 3). The obtained control neutrophils were incubated in five different low doses (2, 4, 6, 8 and 10 nM) (A) and six different normal doses (5, 10, 20, 50, 75 and 100 μM) (B) of MTX for 1 h. The neutrophils were analyzed by the cell viability (MTT) test. Incubation with 100 μM MTX for 1 h was found to be a nontoxic dosage for the neutrophils
Biochemical analysis
The ESR was measured on an Alifax THL 1 ESR analyzer (Alifax SPA, Padua, Italy). Creatinine and ALT were measured using the enzymatic method with a Beckman AU 5800 Autoanalyzer (Beckman Coulter Inc., USA). CRP was measured with a nephelometer (Delta SeacRadim, Pomezia, Italy).
Chemicals
Roswell Park Memorial Institute (RPMI)-1640 medium, caspase 3 substrate (N-acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin, ACDEVD-AMC), penicillin-streptomycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and [3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dihydrorhodamine-123 (DHR-123), fura-2-acetoxymethyl ester (Fura-2/AM), 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide (JC1), and dimethyl sulfoxide (DMSO) were purchased from Molecular Probes (Eugene, OR, USA). Caspase-9 substrate (Ac-Leu-Glu-His-Asp-AMC, AC-LEHD-AMC) was purchased from Bachem (Bubendorf, Switzerland). N-(p-amylcinnamoyl) anthranilic acid (ACA) and fMLP were purchased from Santa Cruz Inc, (Istanbul, Turkey).
Statistical analysis
Data were analyzed using SPSS for Windows version 17.0 (SPSS Inc. Chicago, Illinois, USA). All the results were expressed as mean ± standard deviation (SD). Significant values in four groups were determined using the unpaired Mann-Whitney U test. A p-value of < 0.05 was considered significant.
Results
Demographics and laboratory parameters
The study included 10 patients with RA (4 men, 6 women) and 10 age-matched healthy subjects. All the patients had active RA. Median age was 46.5 years in the patient group and 40.5 years in the control group. Median body mass index (BMI) was 23.8 kg/m2 in the patient group and 25.6 kg/m2 in the control group. Median symptom duration was 7 months in the patient group. No significant difference was found between the two groups with regards to age, gender, BMI, creatinine, and ALT levels. However, ESR and CRP were significantly higher in the patient group than in the control group (p = 0.001 for both). In the patient group, median SDAI score was 31.9, median CDAI score was 31.5 and median DAS-28 score was 5.7 (Table I).
Table I
Age, gender, body mass index (BMI), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor(RF), anti-cyclic citrullinated peptide (anti-CCP), disease activity score-28 (DAS-28); Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), visual analogue scale (VAS) values in controls and RA patients (median (25–75 interquartile ranges) and *p < 0.05 is significant)
Effects of INF and MTX on [Ca2+]I through the inhibition of TRPM2 channels in neutrophils of patients with RA
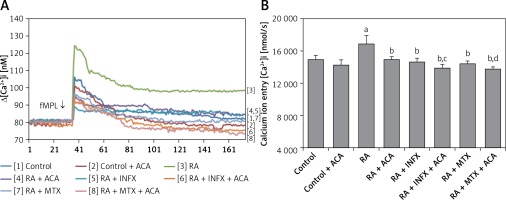
In this study, we aimed to investigate whether the treatment of neutrophils of RA patients with INF and MTX could affect the fMLP-induced Ca2+ mobilization. Figures 2 A and B present the effects of INF and MTX on [Ca2+]i in the neutrophils of the patients. The [Ca2+]i resulting from Ca2+ entry through TRPM2 channel activation in neutrophils was significantly higher in the patient group compared to the control group (p < 0.001). However, the fMLP-induced [Ca2+]i was significantly decreased in the patients who underwent pre-incubation of neutrophils with INF and MTX (p < 0.001). Moreover, [Ca2+]i was further decreased via combinations of INF + ACA and MTX + ACA and was significantly lower in the INF + ACA and MTX + ACA groups than in the INF and MTX groups, respectively (p < 0.05). These findings suggest that the TRPM2 channel is an important channel for Ca2+ entry in the neutrophils of patients with RA.
Figure 2
Effect of infliximab (INF) and methotrexate (MTX) incubation on intracellular calcium concentrations (A and B) of the neutrophils of RA patients (mean ± SD and n = 10). The neutrophils were obtained from both controls and RA patients and were incubated either with infliximab (INF and 0.1 mM for 1 h) or infliximab (MTX and 0.1 mM for 1 h). Fura-2-loaded neutrophils were stimulated with fMLP (1 mM) for 170 s. In some experiments, the neutrophils were incubated with TRPM2 channel blocker, N-(p-amylcinnamoyl) anthranilic acid (ACA 0.025 mM for 10 min)
ap < 0.001 vs. control. bp < 0.001 vs. RA group. cp < 0.05 vs. RA + INF group. dp < 0.001 vs. RA + MTX group.
Effects of INF and MTX on apoptosis, cell viability (MTT), caspase 3 and 9 in neutrophils of patients with RA
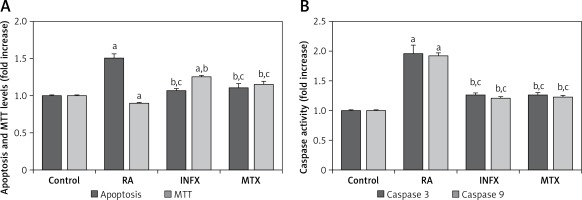
The effects of RA on the programmed apoptosis of neutrophils were evaluated using apoptosis and caspase activation assays. The apoptosis level (Figure 3 A) and the caspase 3 and 9 activities (Figure 3 B) in the patients with RA were significantly higher compared to the controls although the MTT level was lower in the RA group than in the control group (p < 0.001) (Figure 3 A). A significant decrease was found in the apoptosis and caspase 3 and 9 activities of the RA patients whose neutrophils were treated with INF and MTX (p < 0.001), whereas there was an increase in the MTT level in the INF and MTX groups. These findings suggest that INF and MTX have a protective role in the apoptosis of the neutrophils of RA patients because apoptosis can be inhibited by INF and MTX therapies.
Figure 3
Effect of infliximab (INF) and methotrexate (MTX) incubation on apoptosis, cell viability (MTT) levels (A), caspase 3 and 9 (B) activities in the neutrophils of RA patients (mean ± SD and n = 10)
Values were expressed as fold increase (experimental/control). ap < 0.001 and cp < 0.05 vs. control. bp < 0.001 vs. RA group.
Mitochondrial depolarization (JC1) and intracellular ROS production
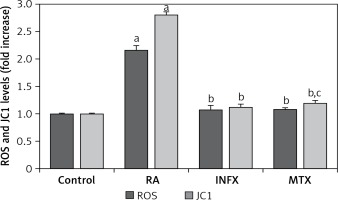
Figure 4 presents the ROS and JC1 results for the RA patients and the healthy controls. Both ROS and JC1 levels were high in the neutrophils of the RA patients compared to healthy controls, whereas the ROS and JC1 levels were significantly lower in the INF and MTX groups as compared to the RA group (p ≤ 0.001).
Figure 4
Effect of infliximab (INF) and methotrexate (MTX) incubation on intracellular ROS and mitochondrial membrane depolarization (JC1) levels in the neutrophils of RA patients (mean ± SD and n = 10)
Values were expressed as fold increase (experimental/control). ap < 0.001 and cp < 0.05 vs. control. bp < 0.001 vs. RA group.
Lipid peroxidation, GSH, and GSHPx
Lipid peroxidation, GSH levels, and GSHPx activity were also investigated as indicators of oxidative stress and antioxidant values. Table II presents the lipid peroxidation, GSH, and GSH-Px values. Lipid peroxidation levels were significantly higher in the RA patients compared to the control group and were significantly lower in the INF and MTX groups compared to the control group (p < 0.05 for both). In addition, the GSH levels and GSHPx activity were significantly lower in the RA group (p < 0.05), whereas a significant recovery was observed in the GSH level and GSH-Px activity in the INF and MTX groups.
Table II
Effects of infliximab (INF) and methotrexate (MTX) on lipid peroxidation, reduced glutathione (GSH) and glutathione peroxidase (GSH-Px) values in the neutrophils of controls and RA patients (mean ± SD and n = 10)
| Parameter | Control | RA | RA + INF | RA + MTX |
|---|---|---|---|---|
| Lipid peroxidation [μmol/g protein] | 16.10 ±1.61 | 18.18 ±0.54a | 15.86 ±1.07b | 15.65 ±0.55b |
| GSH [μmol/g protein] | 6.72 ±0.32 | 6.01 ±0.30a | 6.64 ±0.20b | 6.67 ±0.17b |
| GSH-Px [IU/g protein] | 14.26 ±0.66 | 11.54 ±1.57a | 13.99 ±0.77b | 14.32 ±1.34b |
Discussion
In the current study, we evaluated neutrophils since they have cytotoxic and immunoregulatory functions and act as a source of autoantigens. The results indicated that caspase activity, apoptosis, ROS, and JC1 levels were higher in the RA patients compared to healthy controls. Moreover, a significant decrease occurred in these levels and in [Ca2+]i entry after incubation of the neutrophils with MTX and INF, both of which have been shown in clinical studies to provide effective outcomes in RA treatment.
Calcium signaling cascades play a key role in the activation of immune system cells and in the continuation of immunological events following the activation. [Ca2+]i concentration has been shown to participate in chemotaxis, adhesion, arachnoid acid metabolism, and ROS production [31]. Therefore, some Ca2+ signaling defects have been blamed in the pathogenesis of numerous autoimmune diseases including RA [32]. TRPM2, a member of the TRP family of non-selective cation channels, is a Ca2+-permeable channel embedded in the plasma membrane. TRPM2 also plays a pivotal role in the modulation of basic functions of inflammation including innate immunity response, endothelial barrier integrity, and apoptosis. Additionally, TRPM2 exerts its anti-inflammatory actions via receptor internalization and by preventing neutrophil transmigration through the inactivation of the signal pathway required for the transmigration [9, 33]. To our knowledge, there has been no study in the literature investigating the role of TRPM2 channel or the efficacy of anti-inflammatory drugs in the neutrophils of RA patients. In our study, intracellular [Ca2+]i concentration through modulation of TRPM2 channel activation was greater in the neutrophils of RA patients compared to healthy controls. This finding could be related to the etiopathogenesis of RA or disease activation. On the other hand, a significant decrease was observed in [Ca2+]i concentration after the incubation of neutrophils with MTX and INF, although no significant difference was found between the two drugs. This finding implies that although the two drugs provided similar outcomes, they have different effect mechanisms, suggesting that the TRPM2 channel plays a central role in initiating the inflammatory cascade. Ugan et al. [17] obtained similar findings to our study by evaluating a disease with a different pathogenesis, ankylosing spondylitis, which suggests that the findings obtained in both studies are more associated with the inflammation than the disease. The role of the TRPM2 channel in inflammation may be greater than thought. Moreover, in our study, the decrease in [Ca2+]i concentration became greater when a TRMP2 channel inhibitor (ACA) was added to MTX and INF. TRPM2 channel inhibitors can be used as an adjuvant therapy to standard therapies; however, the resulting molecular response should be clinically supported. Understanding the Ca2+ defects in RA patients and the effect of treatment methods on calcium pathways may lead to the development of novel treatment methods.
Apoptosis is the process of programmed cell death resulting from various physiological pathological conditions. A group of cysteine proteases called ‘caspases’ are responsible for the collapse of the cell into apoptotic bodies during apoptosis. Caspases exist in cells as inactive proenzymes and are activated by proteolytic cleavage. TNF is a strong inducer of apoptosis that uses caspases. Additionally, it plays a key role in cell death by activating mitochondrial ROS production and c-Jun N-terminal kinase after TNF-α stimulation [34, 35]. Although there are at least 14 caspases, caspase 3 is essential for apoptosis and is triggered by free radicals and lipid peroxidation [36]. Genovese et al. [37] reported that caspase 3 led to a significant reduction in apoptosis-regulating proteins and apoptosis following TNF-α inhibition with INF. Another study reported that the INF therapy led to a significant decrease in caspase 3 activity and apoptotic epithelial loss in patients with Crohn’s disease, also noting that the apoptotic epithelial loss that resulted from INF therapy played a key role in the healing of the mucosa [38]. In our study, caspase 3 and 9 activities and apoptosis levels were higher in the neutrophils of RA patients compared to healthy controls and decreased significantly after incubation of the neutrophils with INF. These findings suggest that TNF-α inhibition blocks the apoptotic pathway. Similarly, Shen et al. [39] evaluated a human monocytic cell line and found a significant increase in caspase 3 activity and apoptosis following INF therapy. Weinmann et al. [40] also found that the delayed apoptosis in the neutrophils of RA patients were modulated and increased after MTX therapy. In our study, MTX therapy led to a significant reduction in apoptosis and caspase activity, suggesting that MTX therapy leads to effective suppression of inflammation, thereby normalizing apoptotic pathways.
In patients with RA, ROS production is increased by the activated neutrophils and the ischemia-reperfusion mechanisms of the inflamed joint. Moreover, production of oxidant molecules is increased by disease activation. Modulation of oxidative stress by antioxidant molecules plays a protective role in cartilage and joint damage [7, 41, 42]. Pay et al. [43] reported that the ROS production in neutrophils decreased significantly after the INF therapy. Another study also showed that INF acted as an antioxidant molecule and both oxidative DNA damage and lipid peroxidation decreased after the INF therapy [11]. Similar to INF, MTX has also been shown to result in a significant reduction in oxidant molecules [44–46]. In our study, ROS production, mitochondrial depolarization, and GSH levels were significantly higher in the RA group compared to the control group. Moreover, a significant decrease was observed in the oxidant molecules and a significant increase in the antioxidants. Nevertheless, although no significant difference was observed between the two drugs, both MTX and INF therapies are known to be highly effective and to result in a significant decrease in ROS production, thus shifting the oxidant/antioxidant balance in favor of antioxidants.
Our study was limited in several ways. First, the study had a small patient group and had a cross-sectional design. However, it was determined that the number of patients was sufficient using power analysis before the study started. Another limitation was that other routes of calcium entry, e.g. through membrane voltage/ligand gated calcium channels, were not included in the study. A third limitation is that neutrophil isolation after treatment of patients was not performed and compared with baseline values. The difference between in vitro and in vivo was unknown. Despite these limitations, the inclusion of newly diagnosed and untreated patients in the study is a strength of our study.
In conclusion, MTX and INF, which are two important drugs for RA treatment, reduced calcium ion entry by modulating the TRPM2 channel. Understanding the role of the TRPM2 channel in RA and the inflammatory cascade and the effect of treatment methods on the calcium pathways may lead to the development of novel treatment methods and new pathways in drug mechanisms. Moreover, MTX and INF led to a remarkable reduction in apoptotic cell death and oxidative stress in RA patients, suggesting that these two drugs may have a role in the modulation of the functions of neutrophils in RA patients.


