Introduction
Behçet’s disease (BD) is a deteriorating inflammatory disease in which ulcers (genital and oral), ophthalmic inflammation and skin lesions exist [1]. It can also affect musculoskeletal, nervous, cardiovascular and gastrointestinal systems [2]. The pathogenesis of BD is vague; numerous studies have shown that T cells and monocytes have an important role [3], as they are the predominant types of cells that infiltrate the tissues which are inflamed in BD patients [4, 5]. In addition, disease activity correlates with increased peripheral T cell populations with a pro-inflammatory shift [6, 7].
MicroRNAs (miRNAs) are 19–25 nucleotides in length (short non-coding RNAs). Their transcription suppresses expression of genes via binding target messenger RNA (mRNA), preventing translation and/or inducing degradation [8]. MiRNAs are the main controllers of genes as well as pathways of immune cells such as differentiation, proliferation and apoptosis [9]. Additionally, miRNA dysregulation is related to inflammation and autoimmunity [10, 11].
Many studies have revealed that miR-146a and miR-155 negatively regulated the innate immunity in autoimmune diseases [12, 13]. Moreover, miR-146a and miR-155 expression was documented to be dysregulated in BD [10, 14].
Advances in genetic studies have established a significant relationship between genetic polymorphisms and disease susceptibility as well as response to treatment, allowing early diagnosis and treatment improvement in many diseases [15–19].
Delay in the diagnosis of BD is common as it depends on presence of numerous clinical manifestations. In addition, it is difficult to differentiate BD from other similar diseases. This delay may lead to the occurrence of serious complications such as cardiovascular and pulmonary with high mortality rates. Furthermore, early diagnosis allows use of effective treatment avoiding early permanent damage [20, 21].
Polymorphisms in miRNA, including miR-146a and miR-155, have received great attention in many studies, and they were observed to play a role in susceptibility to autoimmune diseases through affecting the expression of the miRNA targets [22, 23].
These findings suggested a vital role for epigenetic remodeling including miRNA dysregulation in the pathogenesis and the therapeutic response in BD.
The current study was performed to examine the association of miR-146a (rs2910164) and miR-155 (rs767649) polymorphisms with development of BD in the Egyptian population and to examine whether these polymorphisms correlate with the clinical data or the disease activity.
Material and methods
Study participants
Our case–control study recruited 96 patients (12 female, 84male) whose mean age was 33.81 ±7.86 years, from the Rheumatology and Rehabilitation Department, Faculty of Medicine, Fayoum University Hospital. The international criteria for BD were followed in the diagnosis [24]. A total of 100 healthy subjects (21 female, 79 male; mean age: 35.51 ±8.96 years), matched for sex and age, and with negative family history of BD or any other inflammatory-autoimmune disease, were enrolled at the same time from the same geographical zone as control subjects. Each individual provided written consent before participation and after attaining authorization from the Research Ethics Committee in Fayoum, Faculty of Medicine. The study has been amended in accordance with the Helsinki Declaration guidelines.
Behcet’s disease current activity index (BDCAI) was used to evaluate patients’ disease activity status [25].
DNA extraction and detection of genotyping
Entire EDTA blood samples from all participants were used to extract genomic DNA, after being collected in vacuum blood tubes and preserved at –20°C, utilizing the QIA-magnification extraction kit (Qiagen, Valencia, CA) as stated by the manufacturer’s guidelines. DNA samples were quantified using a NanoDrop (ND)-1000 spectrophotometer (Nano Drop Technologies, Inc. Wilmington, USA). Genotyping was achieved by means of RT PCR through the TaqMan allelic discrimination assay (Applied Biosystems, USA) using predesigned primer/probe sets on behalf of miRNA-146a rs2910164 (C/G) [c_15946974_10] and miR-155 rs767649 (A/T)[C_2212229_10]. Probes were manufactured through reporter dye FAM or VIC covalently connected to the 5′ end, whereas quencher dye MGB connected to the 3′ end of the probe (Applied Biosystems, USA). Time of denaturation was 10 min at 95°C followed by 45 cycles at 92°C for 15 s then 60°C for 90 s for annealing and extension. Fluorescence was measured on completion of every cycle and at the endpoint. The Rotor-Gene Q Real Time PCR System (Qiagen, Valencia, CA, and USA) was used to check PCR products.
Statistical analysis
Statistical analyses were performed using SPSS version 25. Distribution of genotypes and frequencies of alleles of BD cases and controls were compared by means of the χ2 test. Analysis of variance (ANOVA) with the multiple comparisons post hoc test or unpaired t test was used for comparisons between groups. The non-parametric Mann-Whitney U test and Kruskal-Wallis test were applied for quantitative variables that are not normally distributed. Logistic regression was done to determine the odds ratio (OR) with 95% confidence intervals and adjust for age and sex as possible confounders. The standard for significance was established at p < 0.05 for all the tests. For analysis of Hardy-Weinberg equilibrium (HWE) the χ2 test was used [26, 27].
Results
Demographic and clinical features of the study groups
The current work included 96 patients with BD (12 female, 84 male) whose mean age was 33.81 ±7.86 years, and 100 healthy control individuals (21 female, 79 male) with an average age of 35.51 ±8.96 years.
The detailed demographic as well as clinical features of the combined patients with BD and healthy subjects are displayed in Table I. Age and gender were matched appropriately.
Table I
Characteristics of BD cases and controls
MiR-146a (rs2910164) and miR-155 (rs767649) genotypes were not in accordance with HWE (p > 0.05) in BD patients. However, in the healthy group rs2910164 genotypes (not rs767649) were in accordance with HWE (p < 0.05).
Frequency distribution concerning the genotypes and alleles of miR-146a (rs2910164) and miR-155 (rs767649) in patients with BD and healthy controls
In comparison to the healthy individuals, there was a significant difference in the frequency of miR-146a (rs2910164) genotypes in BD cases (p < 0.001). The frequencies of GG, CG, and CC genotypes were 29.2%, 66.7%, and 4.2%, respectively, in BD patients and were 10%, 40%, and 50%, respectively, in control individuals. Considering rs2910164 CC genotype a reference group, the frequencies of the GG and CG genotypes were significantly higher in BD patients than control group (p < 0.001 each), and these genotypes remained related to BD after adjustment for age, and sex (adjusted OR = 22.16, 95% CI: 4.73–103.82; p = 0.0001) and adjusted OR = 40.36, 95% CI: 8.93–182.44; p = 0.0002, respectively) (Table II).
Table II
Genotype and allele frequencies of miR-146a (rs2910164) and miR-155 (rs767649) polymorphisms in BD and healthy controls
| Genotype and allele | BD n (%) | Controls n (%) | UnadjustedOR (95% CI) | P-value | AdjustedOR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|
| miR-146a rs2910164 | GG | 28 (29.2) | 10 (10) | 35 (10.05–121.97) | < 0.001* | 22.16 (4.73–103.82) | < 0.001* |
| CG | 64 (66.7) | 40 (40) | 20 (6.71–59.63) | < 0.001* | 40.358 (8.93 –182.44) | < 0.001* | |
| CC | 4 (4.2) | 50 (50) | 1 | 1 | |||
| Dominant model | GG + CG | 92 (95.8) | 50 (50) | 23 (7.85–67.39) | < 0.001* | 31.48 (7.97–124.4 | < 0.001* |
| CC | 4 (4.2) | 50 (50) | 1 | 1 | |||
| Recessive model | GG | 28 (29.2) | 10 (10) | 3.71 (1.69–8.15) | < 0.001* | 1.81 (0.65–4.99) | 0.255 |
| CC + CG | 68 (70.8) | 90 (90) | 1 | 1 | |||
| Additive model | GG | 28 (87.5) | 10 (16.7) | 35 (10.05–121.97) | < 0.001* | 24.46 (5.61–106.73) | < 0.001* |
| CC | 4 (12.5) | 50 (83.3) | 1 | 1 | |||
| Alleles | G | 120 (62.5) | 60 (30) | 3.89 (2.55–5.92) | < 0.001* | 3.67 (2.01–6.67) | < 0.001* |
| C | 72 (37.5) | 140 (70) | 1 | 1 | |||
| miR-155 rs767649 | TT | 28 (29.2) | 15 (15) | 1.867 (0.47–7.49) | 0.379 | 1.24 (0.28–5.42) | 0.776 |
| AT | 63 (65.6) | 80 (80) | 0.79 (0.22–2.84) | 0.715 | 0.51 (0.12–2.05) | 0.342 | |
| AA | 5 (5.2) | 5 (5) | 1 | 1 | |||
| Dominant model | TT + AT | 91 (94.8) | 95 (95) | 0.96 (0.27–3.42) | 0.947 | 0.65 (0.16–2.53) | 0.531 |
| AA | 5 (5.2) | 5 (5) | 1 | 1 | |||
| Recessive model | TT | 28 (29.2) | 15 (15) | 2.33 (1.16–4.72) | 0.018* | 2.32 (1.14–4.73) | 0.021* |
| AA + AT | 68 (70.8) | 85 (85) | 1 | 1 | |||
| Additive model | TT | 28 (84.8) | 15 (75) | 1.867 (0.47–7.49) | 0.465 | 0.99 (0.19–5.11) | 0.988 |
| AA | 5 (15.2) | 5 (25) | 1 | 1 | |||
| Alleles | T | 119 (62) | 110 (55) | 1.334 (0.89–2) | 0.161 | 1.29 (0.86–1.93) | 0.227 |
| A | 73 (38) | 90 (45) | 1 | 1 |
When considering the dominant, recessive as well as additive models of inheritance, comparison of the miR-146a rs2910164 SNP among BD patients in relation to controls revealed an association with increased risk for BD (all p = 0.0001) (Table II).
Also, the G allele of miR-146a (rs2910164) was markedly higher in BD cases than in normal individuals (adjusted OR = 3.67, 95% CI: 2.01–6.67; p = 0.00003).
Regarding miR-155 (rs767649), after using the AA genotype as a reference set, the TT and AT genotypes were not significant risk factors for BD (adjusted OR = 1.24, 95% CI: 0.28–5.42, p = 0.776) and (adjusted OR = 0.51, 95% CI: 0.13–2.05, p = 0.342), respectively.
Moreover, miR-155(rs767649) polymorphism was not associated with BD under the dominant and additive models. However, in the recessive model, the TT genotype was significantly associated with BD and remained associated with BD after adjustment for age and sex (adjusted OR = 2.32, 95% CI: 1.14–4.73, p = 0.021).
Also, no significant difference was detected regarding allele frequency of miR-155 (rs767649) between BD cases and healthy subjects (p = 0.227).
Results of haplotype analysis
We examined the haplotype effect of rs2910164 and rs767649 polymorphisms in patients with BD relative to the control group (Table III). The carriage of combinations of rs2910164 G alleles + rs767649 A allele and rs2910164 G allele + rs767649 T allele were significantly associated with BD (adjusted OR = 6.9, 95% CI: 3.06–15.56, p = 0.0001 and adjusted OR = 3.38, 95% CI: 2.01–5.7, p = 0.0001, respectively).
Table III
Haplotype analysis of miR-146a (rs2910164) and miR-155 (rs767649) polymorphisms in BD compared to healthy controls
| Haplotype | BD n (%) | Controls n (%) | UnadjustedOR (95% CI) | P-value | P-value | P-value |
|---|---|---|---|---|---|---|
| rs2910164 C allele + rs767649 A allele | 40 (20.8) | 80 (40) | 1 | 1 | ||
| rs2910164 G allele + rs767649 A allele | 33 (17.2) | 10 (5) | 6.6 (2.96–14.73) | < 0.001* | 6.9 (3.06–15.56) | < 0.001* |
| rs2910164 C allele + rs767649 T allele | 32 (16.7) | 60 (30) | 1.07 (0.60–1.89) | 0.825 | 1.06 (0.59–1.89) | 0.850 |
| rs2910164 G allele + rs767649 T allele | 87 (45.3) | 50 (25) | 3.48 (2.08–5.82) | < 0.001* | 3.38 (2.01–5.7) | < 0.001* |
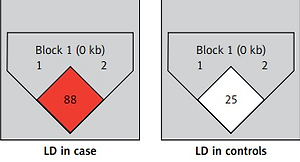
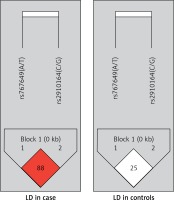
As regards linkage disequilibrium between rs767649 and rs2910164, D’ and r2 values were 0.88 (95% CI: 0.64–0.96) and 0.286, respectively for cases. For controls D’ and r2 values were 0.25 (95% CI: 0.03–0.55) and 0.024, respectively (Figure 1).
Relation between miRNA-146a (rs2910164) and miRNA-155 (rs767649) genotypes and the clinical data of BD patients
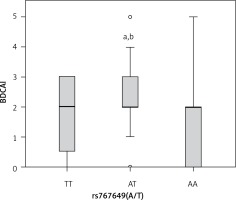
Concerning rs2910164, a significant relationship was documented among patients carrying GG and CG genotypes and degree of BDCAI activity compared with patients carrying CC genotype (p < 0.05, each) (Figure 2).
Figure 2
Box plot representation of Behcet disease current activity index (BDCAI) in different rs2910164 genotypes (GG, n = 28, CG, n = 64, CC, n = 4). Data are expressed as median (interquartile range). aStatistical significance (p < 0.05) compared with CC. bStatistical significance (p < 0.05) compared with CC
Interestingly, the percentage of BD patients with eye manifestations was considerably higher in patients with GG or CG genotypes relative to CC genotype as well as in patients carrying GG genotype compared with CG genotype (p = 0.004). Additionally, the percentage of BD patients who had neurological symptoms was significantly higher in patients carrying GG genotype than in patients with CG or CC genotype (p = 0.018) (Table IV).
Table IV
Effect of miR-146a (rs2910164) and miR-155 (rs767649) on clinical features in BD patients
| Variable | miR-146a (rs2910164) | miR-155(rs767649) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GG | CG | CC | P-value | TT | AT | AA | P-value | ||
| Oral ulcer | Present | 8 (28.6) | 28 (43.8) | 0 (0) | 0.127 | 8 (28.6) | 27 (42.9) | 1 (20) | 0.353 |
| Absent | 20 (71.4) | 36 (56.2) | 4 (100) | 20 (71.4) | 36 (57.1) | 4 (80) | |||
| Genital ulcer | Present | 4 (14.3) | 8 (12.5) | 0 (0) | 1 | 4 (14.3) | 8 (12.7) | 0 (0) | 1 |
| Absent | 24 (85.7) | 56 (87.5) | 4 (100) | 24 (85.7) | 55 (87.3) | 5 (100) | |||
| Skin lesions | Present | 8 (28.6) | 20 (31.2) | 0 (0) | 0.570 | 4 (14.3) | 22 (34.9) | 2 (40) | 0.087 |
| Absent | 20 (71.4) | 44 (68.8) | 4 (100) | 24 (85.7) | 41 (65.1) | 3 (60) | |||
| Arthritis | Present | 0 (0) | 4 (6.2) | 0 (0) | 0.419 | 4 (14.3) | 0 (0) | 0 (0) | 0.024ab |
| Absent | 28 (100) | 60 (93.8) | 4 (100) | 24 (85.7) | 63 (100) | 5 (100) | |||
| Ocular involvement | Present | 16 (57.1) | 16 (25) | 0 (0) | 0.004abc | 8 (28.6) | 22 (34.9) | 2 (40) | 0.754 |
| Absent | 12 (42.9 | 48 (75) | 4 (100) | 20 (71.4) | 41 (65.1) | 3 (60) | |||
| Vascular involvement | Present | 4 (14.3) | 8 (12.5) | 0 (0) | 1 | 4 (14.3) | 8 (12.7) | 0 (0) | 1 |
| Absent | 24 (85.7) | 56 (87.5) | 4 (100) | 24 (85.7) | 55 (87.3) | 5 (100) | |||
| Neurological involvement | Present | 4 (14.3) | 0 (0) | 0 (0) | 0.018ab | 0 (0) | 3 (4.8) | 1 (20) | 0.119 |
| Absent | 24 (85.7) | 64 (100) | 4 (100) | 28 (100) | 60 (95.2) | 4 (80) | |||
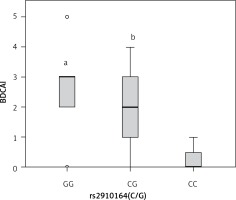
For rs767649, patients carrying AT genotype showed higher BDCAI activity (p = 0.026), compared with TT or AA carriers (Figure 3), while no significant association was found between the rs767649 polymorphism and the other clinical data (all p > 0.05) (Table IV).
Discussion
MiRNAs are evolving as essential regulators of inflammatory immune responses [28]. It has been proposed that genetic variants of miRNAs affect this regulatory role of miRNAs, thereby explaining why a certain variant may contribute to autoimmune or inflammatory diseases [29].
The present study investigated the genotype effects of rs2910164 of miR-146a and rs767649 of miR-155 on BD predisposition and activity in the Egyptian population with BD.
In the present study, we found that the frequencies of the GG and CG genotypes were significantly higher in BD patients than in control subjects. Additionally, the G allele frequency of miR-146a (rs2910164) was found to be significantly higher in BD patients than in normal control subjects.
Consistent with the aforementioned results, in a previous study conducted on the Chinese population, the frequency of the CG genotype was also found to be significantly higher in BD patients when compared with controls. Also they revealed lower frequency of CC genotype as well as the C allele in BD patients than in healthy control subjects. Moreover, they reported higher levels of miR-146a in cases with GG genotype than in cases with CC and GC genotypes. Furthermore, they found significantly reduced levels of miR-146a, IL-7, TNF-α, as well as IL-1β in patients carrying CC than those with GG [14].
Another very recent study in Egypt which enrolled smaller numbers of patients and controls revealed elevation of the incidence of GG genotype, which was not significantly different in the BD patients relative to the controls [30].
Functional studies showed that miRNA-146a is included in certain immune-mediated diseases by regulating multiple pathways involving innate and adaptive immune systems [13]. Attenuation of interleukin (IL)-1 receptor-associated kinase 1 (IRAK1), NF-κB and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) is one of the essential actions of miR-146a [31]. MiR-146a is broadly confirmed to be related to immune diseases including BD [32]. Additionally, the expression level of miR-146 was verified to be significantly upregulated in patients with BD relative to control individuals. It was found that miR-146a expression was related to vascular and ocular manifestations [30].
Furthermore, miR-146a (rs2910164) polymorphism has been previously identified as a risk factor in developing SLE and RA by modulating its expression, affecting the amount of miR-146a, which may subsequently lead to disease development [33, 34].
For rs767649 polymorphism, no significant difference was found in the present study between BD patients and normal control subjects except under the recessive model, where the TT genotype was markedly linked to BD and remained associated with it after adjustment for age and sex.
MiR-155 is one of the early miRNAs that was revealed to be included in the pathogenesis of autoimmune diseases such as RA and SLE [35, 36]. Moreover, this miRNA was reported to be overexpressed in CD4+ T cells in BD patients during disease activity through regulating the Th17 immune response via targeting E26 transformation-specific-1 (Ets-1) [37]. Additionally, Kolahi et al. reported significantly higher levels of miR-155 in patients with BD [38]. They revealed that miR-155 was positively correlated with the levels of TNF-α and negatively associated with CTLA-4.
However, Zhou et al. observed reduced levels of miR-155 in BD patients with active uveitis. They reported that miR-155 constrains the secretion of IL-6 and IL-1b and increases the secretion of IL-10 [10].
Emerging evidence suggests that the rs767649 SNP situated in the upstream region of pre-miR-155 disturbs the binding of regulatory factors including NF-κB to miR-155, and this polymorphism affects the expression of miR-155 [39]. To the best of our knowledge, the association of miR-155 (rs767649) with BD is first studied in this work.
In a study by Ji et al., TT genotype of rs767649 was found to be associated with higher risk and poor survival of patients with hepatocellular carcinoma; they also stated that the T allele contributed to upregulation of miR-155 [39].
Similarly, Xie et al. proposed that TT genotype of the above-mentioned SNP was linked to increased risk of non-small cell lung cancer. They suggested that TT genotype increases miR-155 expression, which contributes to the increased risk of lung cancer [40].
However, Assmann et al. demonstrated that miR-155(rs767649) was related to protection from type 1 diabetes mellitus [41].
In the current study, the rs2910164 GG and CG genotypes were associated with higher disease activity in BD patients, depending on the BDCAI score. Similarly, the same genotypes (GG and CG) were associated with more frequent ocular involvement compared with CC genotype. Moreover, ocular involvement was significantly increased in BD patients carrying GG genotype when compared to CG genotype. Additionally, presence of neurological manifestations was significantly associated with the GG genotype more than CG or CC genotypes.
Previous studies have demonstrated that rs2910164 was associated with high miR-146a expression in numerous autoimmune and inflammatory disorders including BD [14, 34]. It has been shown that this SNP may influence the production of proinflammatory cytokines such as TNF-α, IL-17, and IL-1β, being more highly secreted in BD patients carrying GG genotype than those with CC genotype.
Moreover, it was reported that IL-17 was increased in BD patients and was significantly associated with ocular involvement, disease activity and neurological manifestations [42, 43].
The results of the current study are in agreement with a previous study which concluded that the GG genotype and G allele were markedly associated with pediatric uveitis in Han Chinese [44]. Furthermore, miR-146a level was significantly higher in autoimmune uveoretinitis [45].
Regarding miR-155 (rs767649), the current study further confirmed that patients carrying AT genotype presented with higher BDCAI scores compared with TT or AA carriers. However, there are no reports on the association of the aforementioned SNP with BD.
The following limitations should be considered: First, the present study is a single case-control study with a relatively small sample size, so further studies with large samples in other ethnic groups are required. Second, the possible mechanisms of how these gene polymorphisms affect BD should be evaluated. Finally, the present study cannot rule out that other SNPs located in miR-146a and miR-155 may be associated with BD.
In conclusion, the present study suggested that the miR-146a (rs2910164) and miR-155 (rs767649) polymorphisms are associated with increased risk of BD and can affect disease activity and severity in the Egyptian population.