Introduction
Metabolic syndrome is a highly prevalent and complex disease affecting around 24% of the general population in Europe [1]. It is characterized by the contemporaneous presence of different cardiometabolic risk factors such as overweight, suboptimal blood pressure levels, atherogenic dyslipidemia (low high-density lipoprotein cholesterol (HDL-C) and/or high triglycerides (TG)), dyslgycemia/insulin resistance, and/or systemic inflammation. However, recovering from the condition of metabolic syndrome to a healthy state can significantly reduce the risk of cardiovascular disease [2].
Timely implementation of dietary recommendations and lifestyle modifications is crucial for preventing the development of metabolic syndrome. Maintaining a healthy diet and regular exercise [3, 4] are fundamental to maintain overall metabolic wellness. Nevertheless, supplements properly formulated with scientifically backed ingredients may represent an effective and convenient strategy to support a busy lifestyle. Recent evidence has highlighted a strong correlation between foods rich in flavonoids and cardiometabolic risk reduction [5]. Nutraceutical administration may be useful for controlling altered metabolic parameters in subjects exhibiting features of metabolic syndrome [6, 7]. Among the nutraceuticals and plant extracts believed to be able to modulate metabolism, bergamot is attracting the attention of many researchers for its effectiveness, safety and natural origin [8]. Bergamot and bergamot extracts have been shown to exert a wide range of positive metabolic effects in both preclinical models and clinical tests. In particular, its mechanism of action has been characterized and it has been found that bergamot is able to modulate different metabolic pathways [9]. Therefore, its supplementation may represent a natural multi-target approach for metabolic health. Indeed, bergamot is known to improve cardiometabolic parameters altered in subjects with features of metabolic syndrome such as unbalanced plasma lipid levels, insulin resistance, and systemic inflammation [10].
Most of the studies focus on the purified fraction of bergamot flavonoids [11, 12]. Nevertheless, the whole bergamot phytocomplex is clinically interesting because the different bioactive molecules work in synergy, exerting a stronger effect when compared to the single active fraction.
The aim of our study was to compare the metabolic and vascular effects of 12-week supplementation with two different doses of a new, highly standardized bergamot phytocomplex versus placebo in healthy subjects with unbalanced metabolic parameters.
Material and methods
This was a monocentric, randomized, double-blind, placebo-controlled, three-arm, parallel-group clinical trial conducted on overall healthy volunteers aged 20–75 years with typical features of metabolic syndrome according to the harmonized criteria proposed by the International Atherosclerosis Society [13]. The study was carried out between January and December 2022. In particular, subjects with estimated cardiovascular risk higher than 5% (ESC SCORE) [14], low-density lipoprotein cholesterol (LDL-C) > 190 mg/dl, body mass index (BMI) > 30 kg/m2, with diabetes mellitus, with personal history of atherosclerosis-related cardiovascular disease (CVD) (coronary artery disease, cerebrovascular disease, carotid atherosclerosis diagnosed by ultrasound), myopathy, renal failure, chronic liver disease, or with any other serious or invalidating disease reducing the subjects’ ability to comply with the full protocol, were excluded from the clinical trial. Subjects who were under pharmacological treatment with lipid-lowering drugs, taking medication that affects lipid metabolism, or consuming any food supplements that may potentially impact cholesterol, triglycerides, blood glucose, blood pressure, and liver markers were excluded from the randomization process.
The study was conducted in accordance with the Declaration of Helsinki and with Guidelines for Good Clinical Practice (GCP). The study protocol was approved by the Ethical Committee of the University of Bologna. All volunteers signed a written informed consent document before starting any study related activity.
The study included a 2-week run-in period of diet standardization. After the run-in period, the volunteers were randomized to 3 different groups depending on the treatment with high-dose (700 mg/day) or low-dose (350 mg/day) bergamot extract or to placebo.
The investigational product (produced by AKHYNEX Srl, Polistena, RC, Italy, and commercially available with the tradename of Endoberg by AKHYNEX and as Kalita by Giellepi S.p.A., Milan, Italy) is a water extract obtained from bergamot (Citrus bergamia Risso) fruit cultivated in the Calabrian region, Southern Italy.
The product is produced by, firstly, washing bergamot fruits in steel tanks with water. Then, once peeled, fruits are pressed using a screw press, yielding juice and solid residue (pulp). The pulp obtained is extracted several times with pure water, then dried in rotary kilns and finally sent for pectin extraction for industrial use. The juice obtained by the extraction of the pulp is concentrated in an evaporator in order to obtain a highly concentrated juice suitable for easier storage and transportation. After the addition of arabic gum and adjusting the pH, the concentrated juice is dried in a spray drier, obtaining a powder, referred to below as bergamot phytocomplex.
The water extraction process is capable of preserving all biologically active molecules that make up the phytocomplex, including polysaccharides, amino acids, and polyphenols. These components work synergistically with each other, resulting in an effective extract that does not require further concentration. The product obtained exhibits a unique quali-quantitative profile and contains the full spectrum of bergamot bioactive components, despite not being a highly purified plant extract.
All patients were recommended to take the tested products regularly, i.e. every day. Participants’ adherence was assessed by counting the number of tablets returned at the time of specified clinic visits. At the end of the study all unused tablets were retrieved for inventory.
At enrolment, patients were given standard behavioral and qualitative dietary suggestions to correct their unhealthy habits. In particular, subjects were strongly recommended to follow for the entire duration of the study the general indications of a Mediterranean diet, avoiding an excessive intake of dairy and red meat derived products and reducing dietary excesses, in order to maintain an overall balanced diet. With the advice of a specialist physician, volunteers had to aim to obtain in their diet approximately 50% of calories from carbohydrates, 30% from fat (6% saturated), and 20% from proteins, with a maximum cholesterol content of 300 mg/day and 35 g/day of fibers. Furthermore, the enrolled volunteers were asked to maintain a constant intake of fruits, vegetables, olive oil and wine, in order to reduce the variability in the dietary content of fibers and polyphenols. Individuals were also encouraged to increase their physical activity by engaging in brisk walking or cycling for at least 20 min 3 to 5 times per week [15].
Nutrient intake was estimated from the 4-day records before the randomization and at the end of the intervention period. The nutritional evaluation was performed by an expert nutritionist biologist using Italian Food Composition databases [16].
Assessments
Patients underwent anamnestic evaluations, physical examinations, and laboratory analyses at three time points: baseline, after 6 weeks of treatment, and at the trial’s end (week 12). These measurements followed standardized protocols and were conducted by specially trained staff.
The patients’ personal history was evaluated paying particular attention to CVD and other diseases, assessment of dietary and smoking habits (both evaluated with validated semiquantitative questionnaires) [17], physical activity [18] and pharmacological treatment.
Waist circumference (WC) was measured at the end of a normal expiration, in a horizontal plane at the midpoint between the inferior margin of the last rib and the superior iliac crest. Height and weight were measured to the nearest 0.1 cm and 0.1 kg respectively, with subjects standing erect with eyes directed straight, wearing light clothes, and with bare feet. Then, BMI was calculated as body weight in kilograms, divided by height squared in meters (kg/m2).
The biochemical analyses were carried out on venous blood withdrawn early in the morning from the basilica vein. Subjects fasted for at least 12 h before sampling. The biochemical measurements were centrally performed in our department’s laboratory. Serum used was centrifuged at 3000 RPM for 15 min at ambient temperature. All of the laboratory analyses were performed by trained personnel immediately after centrifugation, in accordance with the standardized methods described in detail elsewhere [19]. The following parameters were obtained or calculated through the appropriate formulae: total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), non-HDL cholesterol (non-HDL-C), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), fasting plasma glucose (FPG), fasting plasma insulin (FPI), homeostatic model assessment of insulin resistance (HOMA-IR), hs-CRP, creatinine (Cr), γ-glutamyl transpeptidases (γ-GT), alanine transaminase (ALT), aspartate transaminase (AST), fatty liver index (FLI), and creatine kinase (CK). LDL-C was obtained by the Friedewald formula (LDL-C= TC – HDL-C – TG/5 expressed in mg/dl) [20]. Non-HDL-C resulted from the difference between TC and HDL-C. HOMA-IR was calculated as the product of FPG and FPI (respectively expressed in mmol/l and U/ml) divided by 22.5 [21]. FLI was obtained by dividing (e0.953 x loge(TG) + 0.139 x BMI + 0.718 x loge(GGT) + 0.053 x WC – 15.745) by (1 + e0.953 x loge(TG) + 0.139 x BMI + 0.718 x loge(GGT) + 0.053 x WC – 15.745) and then by multiplying by 100, where TG are reported in mg/dl and GGT in U/l [22].
Systolic (SBP) and diastolic blood pressure (DBP) were measured in each participant while they were supine and at rest. To do so, a validated oscillometric device was used with cuffs of the appropriate size applied to the right upper arm. Three BP readings were sequentially obtained at a 1-min interval in order to implement detection accuracy protocols. The first one was discarded, and the average between the other two was considered as the study’s variable.
Endothelial function was evaluated through Endocheck (BC Biomedical Laboratories Ltd., Vancouver, BC, Canada), a method embedded within the Vicorder device that guarantees good intra- and inter-operator reliability. The measurement was carried out with patients in supine position and in abstinence from cigarette smoking and caffeinated beverages for at least 12 h. After a 10-min rest, the brachial pulse volume (PV) waveforms were recorded at baseline for 10 s and during reactive hyperemia. The BP cuff was inflated to 200 mm Hg for 5 min and PV waveforms were recorded for 3 min after the cuff was released. Endothelial reactivity (ER) was calculated as change in the PV waveform area, comparing waveforms before and during hyperemia through the equation √PV2/PV1, where PV1 represents PV at the baseline and PV2 represents PV during hyperemia [23, 24].
Safety and tolerability were evaluated through the continuous monitoring of treatment-emergent adverse events, clinical safety laboratory findings, vital sign measurements and physical examinations. A blind, independent expert clinical event committee was appointed by the principal investigator in order to categorize the adverse events that could possibly be experienced during the trial as not related, unlikely related, possibly related, probably related, or definitely related to the study treatment.
Statistical analysis
Data were analyzed using intention to treat by mean of SPSS Statistics version 26.0 (IBM Corporation, Armonk, NY, USA) for Windows. The primary outcome of the study was the change in TG/HDL-C as a marker of atherogenic dyslipidemia in metabolic syndrome. A sample size of 28 subjects per group was needed to detect a mean treatment difference in TG/HDL of 5%, with a power of 0.90 and an α error of 0.05. As per protocol, we decided a priori to check the efficacy of treatments in subjects who had taken at least 90% of the tested product’s doses as per trial design. Normally distributed baseline characteristics of the population were compared using Student’s t test and the χ2 test followed by Fisher’s exact test for categorical variables. Between-group differences were assessed by ANOVA followed by Tukey’s post-hoc test. Data were expressed as mean values with their corresponding standard deviations. All tests were 2-sided. A p level of < 0.05 was considered significant for all tests.
Results
Ninety patients were enrolled and assigned via randomization to receive high-dose (n = 30), low-dose (n = 30) or placebo (n = 30) treatment. All participants completed the trial according to the study design (Figure 1) and no patient experienced any subjective or laboratory adverse event (dropout rate = 0). The adherence to the treatment neared 100% in all groups.
Enrolled subjects maintained similar dietary habits from the randomization until the end of the study, without significant changes in total energy, salt intake or coffee and alcohol consumption (Table I).
Table I
Daily energy and nutrient intakes assessed by patient food diaries, before and after the treatment periods, expressed as mean ± standard deviation
Baseline clinical features and laboratory analyses were similar between groups (Tables II and III).
Table II
Trend in anthropometric and hemodynamic parameters during the trial in the different treatment groups
| Parameter | Placebo arm (n = 30; F = 14, M = 16) | Low-dose arm (n = 30; F = 15, M = 15) | High-dose arm (n = 30; F = 14, M = 16) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week-6 follow-up | Week-12 follow-up | Baseline | Week-6 follow-up | Week-12 follow-up | Baseline | Week-6 follow-up | Week-12 follow-up | |
| WC [cm] | 88.2 ±4.2 | 87.1 ±3.9 | 87.2 ±4.0 | 89.3 ±4.4 | 88.2 ±4.0 | 87.1 ±3.8 | 87.4 ±3.8 | 85.8 ±3.4 | 85.2 ±3.3 |
| BMI [kg/m2] | 27.0 ±1.2 | 26.8 ±1.1 | 26.7 ±1.3 | 26.8 ±1.3 | 26.7 ±1.2 | 26.5 ±1.3 | 26.8 ±1.4 | 26.7 ±1.3 | 26.0 ±1.1 |
| BF (%) | 32 ±3 | 31 ±4 | 31 ±2 | 33 ±4 | 32 ±4 | 32 ±4 | 34 ±4 | 33 ±4 | 31 ±3* |
| SBP [mm Hg] | 128 ±4 | 129 ±3 | 128 ±5 | 129 ±4 | 126 ±3 | 127 ±4 | 125 ±4 | 124 ±5 | 123 ±5 |
| DBP [mm Hg] | 82 ±2 | 83 ±3 | 83 ±2 | 82 ±2 | 85 ±3 | 84 ±2 | 84 ±2 | 83 ±3 | 83 ±3 |
| Endothelial reactivity (%) | 1.38 ±0.21 | 1.31 ±0.19 | 1.35 ±0.20 | 1.33 ±0.24 | 1.37 ±0.21 | 1.39 ±0.19 | 1.36 ±0.17 | 1.39 ±0.22 | 1.44 ±0.15* |
Table III
Trend in laboratory parameters during the trial in the different treatment groups
| Parameter | Placebo arm (n = 30; F = 14, M = 16) | Low-dose arm (n = 30; F = 15, M = 15) | High-dose arm (n = 30; F = 14, M = 16) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week-6 follow-up | Week-12 follow-up | Baseline | Week-6 follow-up | Week-12 follow-up | Baseline | Week-6 follow-up | Week-12 follow-up | |
| TC [mg/dl] | 248 ±14 | 244 ±11 | 243 ±12 | 244 ±12 | 233 ±11 | 228 ±9*° | 250 ±13 | 223 ±10*° | 217 ±9*° |
| LDL-C [mg/l | 164 ±9 | 163 ±9 | 163 ±10 | 162 ±9 | 154 ±7* | 158 ±6 | 169 ±9 | 145 ±8*° | 139 ±7*° |
| HDL-C [mg/dl] | 45 ±4 | 46 ±3 | 44 ±3 | 44 ±2 | 45 ±3 | 46 ±3 | 45 ±3 | 47 ±3 | 48 ±4* |
| Non HDL-C [mg/dl] | 203 ±11 | 198 ±10 | 199 ±10 | 200 ±11 | 188 ±10 | 182 ±10*° | 205 ±12 | 176 ±11*° | 169 ±9*° |
| TG [mg/dl] | 195 ±12 | 177 ±13* | 179 ±11* | 189 ±13 | 170 ±14* | 167 ±13*° | 181 ±14 | 156 ±15*° | 151 ±13*° |
| TG/HDL-C | 4.3 ±1.1 | 3.8 ±1.2* | 4.1 ±1.0 | 4.3 ±1.2 | 3.8 ±1.3* | 3.6 ±1.2*° | 4.0 ±1.3 | 3.3 ±1.4*° | 3.1 ±1.2*° |
| FPG [mg/dl] | 90 ±8 | 88 ±6 | 87 ±7 | 87 ±5 | 85 ±4 | 84 ±4 | 90 ±7 | 87 ±5 | 85 ±5* |
| FPI [µU/ml] | 18.1 ±3.2 | 17.7 ±2.8 | 17.5 ±2.9 | 18.5 ±3.6 | 18.3 ±3.4 | 17.3 ±3.6 | 18.4 ±3.5 | 17.8 ±3.0 | 16.4 ±2.4* |
| HOMA-IR | 4.1 ±0.8 | 3.9 ±0.7 | 3.9 ±0.6 | 3.9 ±0.9 | 3.8 ±0.7 | 3.5 ±0.6*° | 4.1 ±0.7 | 3.9 ±0.8 | 3.6 ±0.7*° |
| AST [U/l] | 22 ±5 | 23 ±4 | 22 ±4 | 19 ±5 | 18 ±3 | 18 ±4 | 22 ±6 | 20 ±4 | 21 ±4 |
| ALT [U/l] | 24 ±6 | 24 ±5 | 23 ±6 | 22 ±4 | 21 ±4 | 21 ±5 | 23 ±5 | 22 ±4 | 23 ±5 |
| γ-GT [U/l] | 29 ±7 | 27 ±7 | 26 ±8 | 26 ±6 | 23 ±5 | 25 ±5 | 27 ±8 | 22 ±6* | 21 ±4*° |
| FLI | 58 ±10 | 57 ±9 | 56 ±9 | 56 ±8 | 54 ±7 | 54 ±8 | 58 ±10 | 54 ±8 | 53 ±8* |
| SUA [mg/dl] | 5.3 ±0.8 | 5.1 ±0.9 | 5.3 ±0.7 | 5.2 ±0.8 | 5.3 ±0.8 | 5.1 ±0.7 | 5.1 ±1.0 | 5.2 ±1.0 | 5.0 ±0.8 |
| CK [U/l] | 131 ±22 | 141 ±38 | 157 ±36 | 144 ±41 | 124 ±36 | 137 ±29 | 139 ±36 | 141 ±38 | 143 ±22 |
| hs-CRP [mg/l] | 2.86 ±0.13 | 2.84 ±0.19 | 2.88 ±0.15 | 2.80 ±0.15 | 2.66 ±0.11 | 2.61 ±0.13 | 2.91 ±0.12 | 2.61 ±0.09* | 2.39 ±0.08*° |
° p < 0.05 vs. placebo. ALT – alanine aminotransferase, AST – aspartate aminotransferase, CK – creatine kinase, FPG – fasting plasma glucose, FLI – fatty liver index, FPI – fasting plasma insulin, γ-GT – γ-glutamyl transferase, HDL-C – high-density lipoprotein cholesterol, HOMA-IR – homeostatic model assessment for insulin resistance, hs-CRP – high-sensitivity C-reactive protein, LDL-C – low-density lipoprotein cholesterol, SUA – serum uric acid, TC – total cholesterol, TG – triglycerides.
Regarding the primary outcome of the study, the TG/HDL-C ratio improved in all treatment groups vs. baseline after 6 weeks of treatment (p < 0.05), while it improved versus placebo in both the low-bergamot dose and high-bergamot dose groups (p < 0.05).
In the placebo group, only plasma TG significantly decreased versus baseline values after 6 and 12 weeks (p < 0.05), and TG/HDL-C ratio after 6 weeks (but not after 12 weeks). No other parameter changed during the observation period.
In the low-dose treated group, after 6 weeks plasma LDL-C and TG significantly decreased versus baseline values (p < 0.05). After 12 weeks, TC, non-HDL-C, TG and HOMA-index improved both versus baseline and placebo values (all, p < 0.05).
In the high-dose treated group, after 6 weeks TC, LDL-C, non-HDL-C, TG, γGT and hsCRP significantly decreased versus baseline values (p < 0.05). TC, LDL-C, non-HDL-C, and TG also decreased versus placebo values (p < 0.05).
After 12 weeks, TC, LDL-C, non-HDL-C, TG, FPG, FPI, HOMA-Index, GGT FLI, and hsCRP significantly decreased versus baseline values (p < 0.05), while HDL-C increased. TC, LDL-C, non-HDL-C, TG, HOMA index, GGT, and hsCRP values significantly decreased versus placebo, as well (p < 0.05).
Percentage body fat decreased by 9.7 ±1.1% after 12 weeks of treatment versus baseline only in the high-dose treated group (p < 0.05). Similarly, endothelial reactivity (ER) improved by 0.08 ±0.02% after 12 weeks of treatment versus baseline only in the high-dose treated group (p < 0.05).
None of the other anthropometric or hemodynamic parameters exhibited a significant difference compared to the baseline or placebo (p always > 0.05).
Discussion
There is an increasing interest in clinically tested nutraceuticals with positive effects on human metabolic health, in particular when they can simultaneously act on different pharmacological targets [6, 25]. Among these nutraceuticals, bergamot extracts seem to be among the most promising [26].
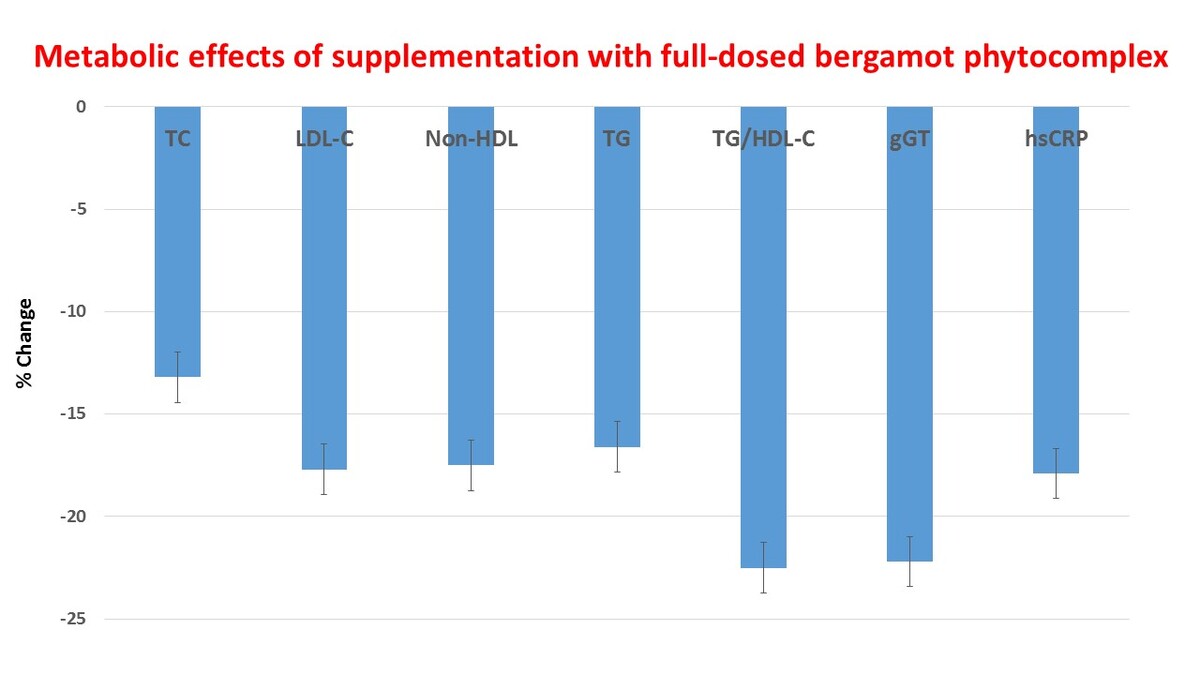
Overall, the treatment tested in the present randomized, double-blind, placebo-controlled, three-arm, parallel-group clinical trial showed a general improvement of metabolic parameters in adults subjects with features of metabolic syndrome. We observed that after 12 weeks of treatment, both tested bergamot doses were able to improve significantly both atherogenic dyslipidemia and insulin sensitivity versus placebo. In the high-dose treated group, TC, LDL-C, non-HDL-C, and TG improved versus placebo already after 6 weeks of treatment. After 12 weeks of treatment, TC decreased by 13.2 ±2.1%, LDL-C by 17.7 ±3.2%, non-HDL-C by 17.5 ±3.1%, TG by 16.6 ±3.3%, TG/HDL-C by 22.5 ±4.4%, HOMA-IR by 12.2 ±2.1%, GGT by 22.2 ±4.6%, and hsCRP by 17.9 ±3.4% versus baseline (p < 0.05) and versus placebo (p < 0.05).
The observed effects on lipid metabolism are consistent with those reported in a recent systematic review of randomized clinical trials that evaluated the efficacy of bergamot extract. These trials showed that the extract can lead to significant decreases in TC, ranging from 12.3% to 31.3%, as well as in LDL-C and TG, with reductions ranging from 7.6% to 40.8% and 11.5% to 39.5%, respectively. Notably, the largest reductions were seen at doses double the highest one we tested [27].
Our study found that high-dose treatment with a bergamot phytocomplex led to an improvement in percentage body fat compared to baseline, but not compared to placebo. Additionally, although not statistically significant, a slight reduction in waist circumference of approximately 2.2 cm was observed in the high-dose treatment group. These findings suggest that the insulin sensitivity improving properties of bergamot extract may have contributed to this effect [28].
Endothelial reactivity improved versus baseline in the high-dose treated group, which could be related to a direct positive impact of Citrus polyphenols on endothelial function [29].
All of these data are in line with observations reported for the new improved bergamot-based nutraceutical products with higher polyphenol levels recently tested and not yet included in the previously cited meta-analysis [11, 30].
Unlike most of the nutraceutical bergamot products on the market, the extract used in the present investigation is not selectively purified in order to contain high levels of polyphenols, preserving the natural bergamot phytocomplex. In fact, preliminary pre-clinical data [31] show that the tested bergamot phytocomplex contains multiple bioactive components and in particular soluble fibers, polyphenols and small molecules, such as stachydrine. LC-MS analysis identified 86 compounds, with hesperetin, naringenin, apigenin and eriodictyol glucosides being the main components found. Endoberg/Kalita contains the full spectrum of bergamot bioactive molecules which, acting synergistically [31], may justify the observed efficacy found in both preclinical and clinical studies. We speculate that such efficacy is due to the whole phytocomplex and particularly the pectin contents, which may assist and enhance the bioavailability of active components [32].
The findings of the present clinical investigation highlight that the bioactive components present in the bergamot phytocomplex work in synergy to enhance clinical effectiveness. We can speculate that while the flavonoid fraction plays a significant role, other classes of molecules and secondary metabolites may also contribute to this effect, suggesting that the active molecules responsible for the observed benefits are diverse and not limited to a single class of compounds. Given the lack of bioactive compounds acting on 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase, it could be considered in the management of statin intolerant subjects with a low added cardiovascular risk [33].
We have to acknowledge some study limitations. In particular, the trial was short, so we cannot infer that longer intake of the tested dietary supplement could have led to an eventual further improvement of the studied parameters or to a reduction of the positive effect observed. Moreover, the sample size of the study was relatively small, even if adequately powered for the aim of the trial. For this reason, we did not conduct any advanced statistical analysis, such as regression, to identify predictors of better or worse responses to the tested product. Furthermore, as nutraceuticals need to be taken for extended periods, ensuring their safety has become a topic of growing importance [34, 35]. With this in context, the overall middle-term tolerability of the nutraceuticals tested has been largely confirmed by the available literature and by the present trial. Further long-term data on a larger patient sample should be obtained in a new double-blind randomized clinical trial in order to confirm our present findings.
In conclusion, in our study the Endoberg/Kalita bergamot extract was found to significantly improve glucose and lipid metabolism, as well as inflammation, in individuals with metabolic syndrome. These findings suggest that this natural extract could be a promising multi-target solution for enhancing cardiometabolic health.