Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by resting tremors, muscle rigidity, and bradykinesia (slowness of movement) [1]. According to the World Health Organization (WHO) 2023 report, the disability-adjusted life years (DALYs) and the number of deaths attributed to PD have increased by 81% and 100%, respectively, since the early 20th century [2]. As of 2021, PD affected more than 6 million individuals globally, and a cure or preventive treatment for the disease remains elusive [3]. Furthermore, the majority of PD cases are sporadic, underscoring the need for increased research efforts to identify non-genetic factors in its development [4].
The gut microbiota plays a significant role in influencing the risk of developing PD [5–7]. Additionally, trimethylamine N-oxide (TMAO), a microbial amine metabolite originating from dietary components, can be detectable in the cerebrospinal fluid, suggesting its potential involvement in the development of neurodegenerative diseases [8–10]. However, studies investigating the potential use of circulating concentrations of TMAO and its precursors as biomarkers or therapeutic targets for PD have yielded conflicting results due to confounding factors. Thus, it remains uncertain whether circulating concentrations of TMAO and its precursors are linked to the risk of PD. Therefore, further research is needed to clarify this association.
Mendelian randomization (MR) analysis utilizes genetic variations as instrumental variables (IVs) to investigate the causal relationship between an exposure and an outcome, leveraging the strong association between IVs and exposure factors [11]. This approach offers a more precise assessment of the impact of an exposure factor on the risk of disease occurrence compared to other analyses by reducing bias arising from reverse causality [12]. Additionally, employing appropriate published genetic data as IVs facilitates efficient MR analyses, as they reutilize previously obtained data and yield novel insights [13].
In this study, we conducted a comprehensive assessment of the relationship between circulating concentrations of TMAO and its precursors and the risk of PD. We utilized a two-sample MR analysis, which involved aggregating data from three genome-wide association study (GWAS) databases: the International Parkinson’s Disease Genomics Consortium (IPDGC), Parkinson’s Research: The Organized Genetics Initiative (PROGENI) and GenePD, and FinnGen. For this analysis, we utilized relevant single-nucleotide polymorphisms (SNPs) as genetic instrumental variables (IVs).
Material and methods
Data source
All the data were obtained from previously published GWAS. The SNPs associated with circulating concentrations of TMAO, betaine, carnitine, and choline, along with their respective summary data, were retrieved from the study conducted by Rhee et al. [14]. The PD data were sourced from the IEU OpenGWAS database, which is an extensive epidemiological unit of the Medical Research Council (https://gwas.mrcieu.ac.uk). We carefully organized and summarized the available research data, ensuring the independence of the study populations. Three databases were selected for the present study to investigate the relationship between circulating concentrations of TMAO, betaine, carnitine, choline, and the risk of PD. Other databases were excluded due to factors such as redundant population samples, an excessive admixture between European and Asian populations, and considerations. The selected datasets comprised a total of 36,652 cases and 666,542 controls (Supplementary Table SI), with no between-sample overlap in terms of exposure and outcome. The original studies included in these datasets had obtained ethical approval from their respective institutions. As our study relied solely on publicly available data from these studies, no additional ethical approval was needed. Furthermore, the analysis was limited to populations of European ancestry to minimize potential confounding factors associated with race.
IVs
The independent variables (IVs) were determined for each metabolite using the subsequent screening process.
A significance level of p < 5 × 10–5 was employed as the inclusion criterion to guarantee the attainment of statistically significant associations.
Linkage disequilibrium (LD) analysis was conducted for every significant SNP associated with the circulating concentrations of each metabolite. The analysis followed the procedure outlined by the European 1000 Genome Project Reference Group [15] and implemented stringent default parameters (r2 = 0.001, kb = 10,000). In cases where LD was identified, only the SNP with the lowest (i.e., most significant) p-value was retained as an independent genetic variant.
MR analysis
Following the association analysis of each SNP, we calculated the Wald ratio. The Wald ratios were then combined and evaluated using the inverse variance weighted (IVW) method. Specifically, each SNP’s Wald ratio was weighted by the inverse of its IV variance in order to obtain an overall IV-based estimate of causality. This approach allowed us to disregard any invalid genetic IVs and perform a weighted regression analysis [16]. Additionally, we utilized an MR-Egger regression to validate and assess the stability of our MR results. The intercept of an Egger regression serves as an indicator for the presence of pleiotropy, with a non-zero intercept suggesting the possibility of horizontal pleiotropy, which could introduce bias in the IVW results [17].
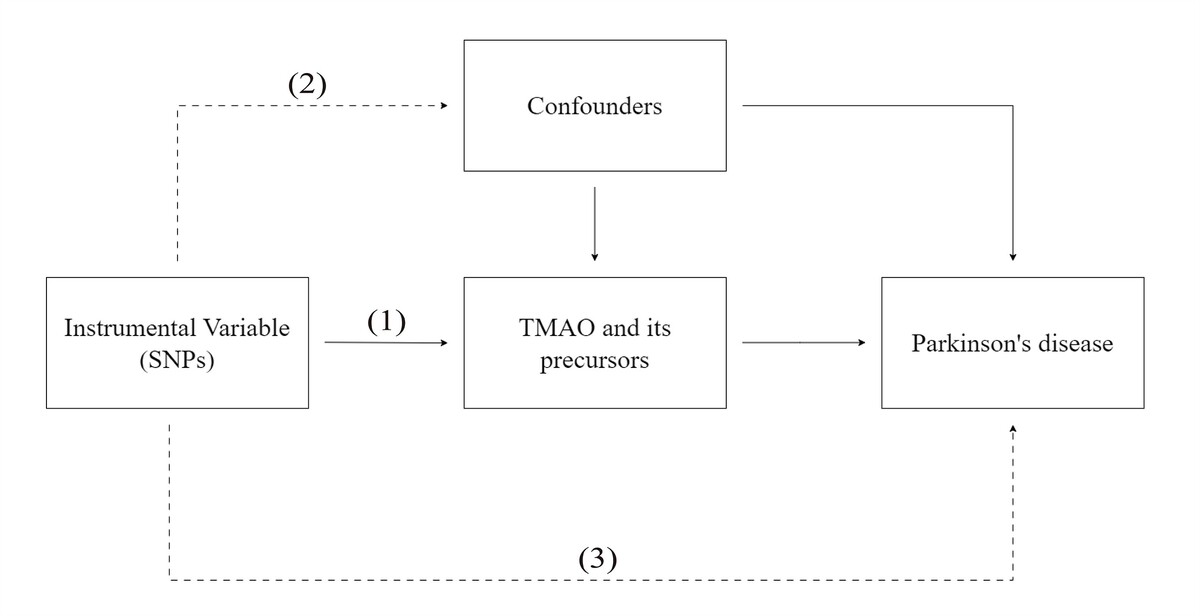
As shown in Figure 1, the IVs in this MR analysis were required:
Figure 1
Graphical representation of the MR assumptions, i.e. (i) relevance, (ii) independence, (iii) exclusion restriction, in a two-sample MR design. The continuous lines represent the relationships that hold in MR analysis. Dashed lines depict the association that should not be present to satisfy the second and third assumptions. SNP–exposure associations are derived in Sample 1, and SNP–outcome associations in Sample 2
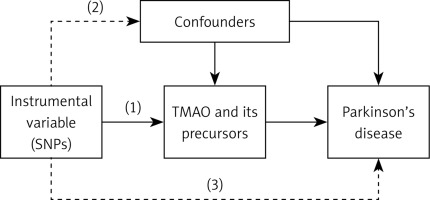
to be associated with the levels of the exposure factor;
to be independent of any confounding factors related to the exposure and PD;
to avoid influencing PD through pathways other than the exposure, it is important to adhere to specific measures [18].
All analyses were performed using the TwoSampleMR package in R version 4.1.3 [19]. The statistical significance level was defined as p < 0.05. Details of the package can be found at https://mrcieu.github.io/TwoSampleMR/reference/clump_data.html.
Sensitivity analysis
The reliability of the results was assessed using the leave-one-out method. In this approach, each SNP was systematically removed one by one to determine whether any of them acted as outlier IVs, exerting a strong influence on the estimated causal effect. If the removal of SNPs individually had minimal impact on the results, it indicated that our findings were robust and not heavily dependent on any single IV.
Results
IVs
We identified a total of 51, 29, 29, and 42 SNPs associated with the levels of TMAO, choline, carnitine, and betaine, respectively, in the circulation. All of these SNPs achieved the significance threshold for genome-wide exposure analysis (p < 5 × 10–5, r2 < 0.001, and kb = 10,000), which is widely accepted in the field. To ensure the validity of our findings, we calculated the F-statistic for each SNP and found that all of them exceeded the value of 10, indicating that they were not likely to introduce weak instrument bias (Supplementary Table SII).
MR analysis
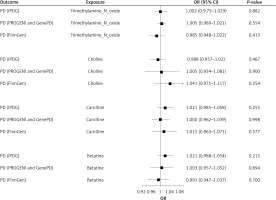
The MR analysis conducted on data obtained from the three databases revealed no significant causal relationship between circulating concentrations of TMAO and its precursors and the risk of PD (all p > 0.05) (Figure 2). This finding was corroborated by other analytical approaches, such as sample mode, weighted mode, weighted median, and MR-Egger regression, all of which yielded non-significant results (all p > 0.05) (Supplementary Table SIII).
Assessment of the relationship between circulating concentrations of TMAO and its precursors and the risk of PD in the IPDGC database
The primary analysis using the IVW method in MR did not find any evidence of a causal relationship between circulating concentrations of TMAO and its precursors, namely choline, carnitine, and betaine, and the risk of PD. The results of the analysis showed that the odds ratios (ORs) and 95% confidence intervals (CIs) for TMAO–PD, choline–PD, carnitine–PD, and betaine–PD were 1.002 (0.975–1.029, p = 0.882), 0.988 (0.957–1.020, p = 0.467), 1.021 (0.985–1.058, p = 0.254), and 1.021 (0.988–1.054, p = 0.215), respectively. Additional analytical methods also supported these findings, confirming that there was no significant causal association between circulating concentrations of TMAO and its precursors and the risk of PD in the population under study, which was based on the data obtained from the International Parkinson Disease Genomics Consortium (IPDGC) database (all p-values > 0.05; Supplementary Table SIII).
Assessment of the relationship between circulating concentrations of TMAO and its precursors and the risk of PD in the PROGENI and GenePD database
The primary IVW analysis conducted using MR did not find any evidence to support a causal relationship between circulating concentrations of TMAO and its precursors and the risk of PD. The results of the analysis are as follows: TMAO–PD: OR = 1.005 (95% CI: 0.989–1.021), p = 0.554; choline–PD: OR = 1.005 (95% CI: 0.934–1.081), p = 0.900; carnitine–PD: OR = 1.000 (95% CI: 0.962–1.040), p = 0.998; and betaine–PD: OR = 1.003 (95% CI: 0.957–1.052), p = 0.894. Furthermore, additional analytical methods confirmed that there was no statistically significant causal association between TMAO and its precursors and the risk of PD in the population studied, based on data obtained from the PROGENI and GenePD databases (all p > 0.05; Supplementary Table SIII).
Assessment of the relationship between circulating concentrations of TMAO and its precursors and the risk of PD in the FinnGen database
The primary IVW analysis using MR did not find any evidence supporting a causal relationship between circulating concentrations of TMAO and its precursors and the risk of PD. Specifically, the ORs and 95% CIs for the relationships TMAO–PD, choline–PD, carnitine–PD, and betaine–PD were as follows: 0.985 (0.980–1.022, p = 0.413), 1.041 (0.971–1.117, p = 0.254), 1.015 (0.963–1.071, p = 0.577), and 0.991 (0.947–1.037, p = 0.700), respectively. These results do not support the presence of causal relationships between circulating concentrations of TMAO and its precursors and the risk of PD in the population. Furthermore, the findings of other MR analyses also support this conclusion (Supplementary Table SIII).
Sensitivity analysis
Cochran’s Q test was conducted to evaluate heterogeneity, indicating no significant heterogeneity among the studies (Table I). The MR-Egger regression analysis detected no evidence of horizontal pleiotropy for any of the SNPs, with all p-values exceeding 0.05 (Table I). Moreover, the leave-one-out sensitivity analysis demonstrated that no individual SNP exerted a substantial influence on the overall findings (Supplementary Table SIV). Taken together, these findings underscore the robustness of the MR analysis results, indicating minimal impact from both heterogeneity and horizontal pleiotropy.
Table I
Heterogeneity and pleiotropic tests of Mendelian randomization analysis
Discussion
This study employed a two-sample MR analysis to examine the potential causal association between circulating levels of TMAO, choline, carnitine, betaine and the risk of PD in populations represented by one of three PD-related GWAS databases. The findings of this study do not support a causal relationship between circulating concentrations of TMAO and its precursor molecules and the risk of PD. Nonetheless, it is important to exercise caution when interpreting these MR results, as such a relationship has not been independently evaluated in other investigations. Consequently, further research and validation efforts are necessary to ascertain the presence or absence of such a relationship.
The association of circulating concentrations of TMAO and its precursors with the risk of PD has been investigated in other studies. However, these studies were influenced by reverse causality and confounding factors. For instance, it was observed that circulating concentrations of TMAO were elevated in PD patients, independently of disease characteristics, treatment status, and lifestyle factors [20]. This increase in concentration is primarily driven by significant variations in the gut microbiota, which strongly affect plasma concentrations of TMAO [21, 22]. Additionally, concentrations of TMAO and its precursors, such as choline, betaine, and carnitine, can be influenced by variations in dietary habits among different population groups, including cases and controls. For example, low calorie intake in PD patients is often attributed to difficulties in swallowing, refusal to eat, and digestive problems [23]. Due to these factors, establishing a definitive causal relationship between the gut microbiota and PD remains challenging.
Concentrations of TMAO in the cerebrospinal fluid of individuals with mild-to-severe cognitive impairment and Alzheimer’s disease have been found to suggest the involvement of TMAO in the decline of neurological function [24]. Additional research has indicated that TMAO can contribute to mitochondrial damage, the formation of superoxides, and the promotion of pro-inflammatory factors, thus leading to increased expression, accumulation, and epigenetic changes of aging markers. As a result, the risk of PD may potentially increase, suggesting a role of TMAO in neurodegeneration and its contribution to the risk of PD [25, 26]. Conversely, other studies have suggested that TMAO plays a protective role in PD. Higher concentrations of TMAO may alleviate neurodegeneration in PD and predict favorable clinical outcomes in the early stages of the disease [27]. Moreover, TMAO has been found to counteract endoplasmic reticulum stress by promoting proper protein folding, which can reduce the formation of toxic aggregates within cells and potentially have neuroprotective effects in PD [28–30].
Choline, an essential nutrient for all cells, plays a crucial role in the biosynthesis of cellular membrane phospholipids [31]. While anticholinergic drugs have proven effective in managing PD symptoms, the precise mechanisms underlying choline’s action in PD remain unclear [32, 33]. Several studies have highlighted the necessity of choline for the biosynthesis of choline-containing phospholipids and proposed its significant neuroprotective effects, as it is required for the synthesis of acetylcholine precursors [34, 35]. Furthermore, choline-containing phospholipids have been utilized as therapeutic agents in combination with levodopa for PD treatment [36]. However, some investigations have suggested choline as a potential risk factor for PD due to excessive choline intake, which can result in elevated lipid concentrations in the blood. Both fat and free fatty acids have been implicated in promoting neuronal damage [37–39].
Carnitine plays a protective role in PD through various mechanisms. Firstly, it promotes the formation of primary cilia in cells, inhibiting mitochondrial fragmentation and excessive production of reactive oxygen species within the mitochondria, Additionally, it enhances mitochondrial function and reduces the production of pro-inflammatory cytokines. Bae et al. have extensively researched these effects of carnitine in PD [40]. Furthermore, carnitine’s involvement in β-oxidation in primary metabolism leads to increased energy production and supports the urea cycle, which aids in the elimination of ammonia. This process helps slow down the progression of neurodegenerative diseases [41]. However, it is important to note that studies by Jiménez-Jiménez et al. and Zhou et al. have reported no significant evidence for an association between carnitine and the risk of PD [39, 42].
Choline serves two primary functions in the human body. Firstly, it acts as a major osmolyte in tissues, regulating cell volume. Secondly, in the kidneys, choline acts as a methyl donor, converting the toxic metabolic product homocysteine into methionine [43, 44]. Accumulation of nitric oxide and homocysteine is strongly associated with the development of neurodegenerative diseases, such as PD [45, 46]. Choline has been shown to reduce blood homocysteine levels and enhance the expression of memory-related proteins [47]. Furthermore, an animal experiment demonstrated that choline inhibited nitric oxide production by microglial cells [48]. However, it is worth noting that choline has also been linked to elevated blood lipid concentrations [37, 49] and may worsen motor symptoms in PD patients, as evidenced by increased Unified Parkinson’s Disease Rating Scale Part III scores [39].
Previous studies investigating the relationship between TMAO and its precursors and the risk of PD have been limited due to the presence of confounding factors, which has made it challenging to establish a definitive causal relationship. In order to overcome this limitation, we employed an MR analysis in the present study. This method is less susceptible to the influence of confounding factors such as socioeconomic status [50]. By utilizing publicly available GWAS summary data, our study benefits from more accurate results obtained from a broader range of databases and larger sample sizes when compared to previous investigations. To address potential bias arising from sample overlapping across databases, we employed the two-sample weighted IVW method. Moreover, we incorporated other analytical techniques to assess the consistency of causal associations, thereby reinforcing the robustness of our findings.
However, this study has several limitations. Firstly, the samples were exclusively drawn from European populations, which may limit the generalizability of our findings to non-European populations. Secondly, while MR analysis helps to minimize the impact of confounding factors, it cannot completely remove the influence of genetic pleiotropy. Therefore, we may not have fully assessed potential causal associations between certain exposure factors and the risk of PD. Thirdly, MR analysis only evaluates linear relationships, overlooking potential non-linear associations between exposure factors and the risk of PD. These limitations emphasize the importance of interpreting our results with caution and underline the need for further investigations in diverse populations, including exploration of non-linear relationships between exposure factors and the risk of PD.
In conclusion, in this study we conducted a comprehensive analysis of data from multiple GWAS databases to investigate the potential causal relationship between circulating concentrations of TMAO and its precursors and the risk of PD. The results of our analysis did not yield conclusive evidence in support of a direct causal relationship between TMAO and PD.