Introduction
In the early wave of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic, Italy reported a higher number of recorded cases and deaths than other European countries. By 31 May 2020, a total of 232,639 confirmed cases and 32,981 deaths had been reported, indicating a standardized mortality rate of 46.8 per 100,000 inhabitants [1].
The clinical presentation of the disease at the outbreak of the pandemic was very heterogeneous nationwide. In Northern Italy, the disease spectrum showed more severe signs characterized by a high fatality rate, frequent admissions at intensive care units (ICUs), older patients and a higher number of comorbidities [2, 3]. Conversely, the early few epidemiologic studies in Southern Italy [4, 5] suggested that the infection spread at a lower rate and less severely than in Northern Italian Regions [6, 7]. The heterogeneous distribution of SARS-CoV-2 infection across Italian regions could be related to different environmental conditions, such as the past and cumulative exposure to particulate matter pollution [8].
Emerging evidence shows that older people [9] and people with multimorbidity present a more severe disease spectrum, in both cases of clinical assessment based on patients’ clinical symptoms, signs, and chest imaging manifestations [10] and in case of occurrence of ICU admission and death [11]. It has been documented that elderly patients have the highest mortality rates [9], show a significantly higher risk of developing acute respiratory distress syndrome (ARDS) [12] and progressing towards death [3, 13].
The majority of primary studies concerning the clinical characteristics and prognosis of patients with SARS-COV-2 are retrospective cohorts, with clinical information only evaluated at the baseline [14–16] or used as a stratification variable of the survival experience of the cohort [17]. Only a few hospital-based studies take full advantage of the cohort design by tracking patients’ clinical and laboratory parameters from admission to discharge. Some of these studies consider the D-dimer trend [18], the temporal evolution of C-reactive protein concentration and lymphocyte count [19], the variation of haematological and immunologic biomarkers in patients with SARS-COV-2 infection across three time periods [20], the clinical courses of major symptoms, the outcomes and the viral shedding [21].
With this retrospective longitudinal cohort study, we propose to fill in two knowledge shortfalls:
– to generate evidence about the mortality risk for SARS-COV-2 disease, relating to epidemiological and clinical factors, therapy and clinical course, in a sample of elderly patients during the early wave of the pandemic across Southern Italy,
– to suggest prognostic indicators based on the day-to-day follow-up of clinical and laboratory findings.
Material and methods
Study design and participants
We performed a retrospective longitudinal cohort study of all patients admitted for SARS-CoV-2 infection at Partinico COVID Hospital in the province of Palermo (Sicily, Southern Italy) between 4 March and 25 April and followed up until 31 May 2020. Patients referred to this centre came from the emergency departments of other hospitals in Palermo with a confirmed diagnosis of SARS-CoV-2 infection. Diagnosis of SARS-COV-2 was confirmed by a positive real-time reverse transcriptase PCR from nasal swabs and analysed by the molecular virology unit according to the WHO guidelines and protocol by Corman et al. [22]. Patients were followed up day-to-day until death or hospital discharge. The criterion for discharge was the occurrence of two consecutive nasal and pharyngeal swabs, at an interval of at least 24 h, negative for SARS-CoV-2 RNA.
A multidisciplinary team made of an infectious physician, a pulmonologist, a cardiologist, a diabetes specialist, a haematologist, an anaesthesiologist and an internist was responsible for the clinical management of SARS-COV-2 patients. Psychiatric and psychological support was available for hospitalized patients and the working medical team.
Ethical statement
The research was conducted according to the Helsinki Declaration. All patients signed the informed consent upon admission to the hospital. The study protocol received the ethical approval from the local Ethical Committee (Azienda Ospedaliera Policlinico “Paolo Giaccone” of Palermo, No. 4/2020, dated 22 April 2020).
Data collection
We collected demographic data (sex and age), epidemiological information, clinical data, laboratory tests and treatment data. The data were extracted from medical records, and the epidemiologic information was obtained by the clinical history and by the medical interview carried out during patients’ hospitalization.
The epidemiological information included i) recent exposure to people with confirmed SARS-CoV-2 infection, ii) coming from a recent journey abroad or in other Italian regions, iii) living in a residential care home, and iv) having a history of previous hospitalizations. Clinical symptoms were collected according to the European CDC definition [9].
We included data and information about the underlying chronic medical conditions that increase the risk of severe SARS-COV-2 disease [9]. Specifically, they are diabetes, chronic kidney failure (CKF), hypertension, heart conditions and other cardiovascular and cerebrovascular diseases (CVD), chronic obstructive pulmonary disease (COPD), dyslipidaemia, autoimmune disease, neurologic conditions such as dementia [23] and mental disorders [24], glaucoma, asthma and cancer. In patients aged over 65, a multidimensional geriatric examination, including a cognitive and functional assessment, was performed to assess dementia. Multimorbidity was defined as the coexistence of several conditions, without any of them taking predominance over the others. Accordingly, we defined multimorbidity as two or more chronic diseases in the patient [25].
The treatment data collection included: antivirals, hydroxychloroquine, tocilizumab or immunomodulatory and other adjunctive therapies for COVID-19 such as oxygen support, antibiotic drugs, steroid treatment and enoxaparin prophylaxis and treatment. The treatment prescription was based on national guidelines in use at enrolment time and validated by the WHO [26].
All data were checked by two physicians (DD and DB), and a third physician (PD) decided upon any interpreting differences between the two primary reviewers. Because of clinicians’ high workload, three medical statisticians from the University of Palermo performed raw data extraction, recording and data analysis (DM, LM and ME).
Statistical analysis
Categorical variables were summarized as counts and percentages, discrete and continuous variables as medians and interquartile range (IQR). In the presence of an asymmetric distribution, quantitative variables were log-transformed. Pearson’s correlations were calculated between pairs of quantitative variables. The differences between non-survivors and survivors were assessed through the Kaplan-Meier method and the proportional hazards (PH) Cox model. The PH Cox model was implemented using the “survival” package in the R environment (version 3.5.3). Due to the longitudinal nature of the data and the presence of time-varying variables, the time-dependent data set was built up according to the time-interval format [27], and the “coxph” function was used to estimate the parameters. This model at each event time compares the current covariate values of the subject who had the event to the current values of all others who were at risk at that time. Explanatory significant variables at univariable analysis were chosen as candidate prognostic factors for multivariable analysis. Moreover, a maximum of four explanatory variables was included to avoid overfitting. Moreover, variables containing more than 5% missing values were not considered in multivariable analysis. ROC-based threshold analysis was used to calculate the optimal cut-off value for laboratory findings, when appropriate. The cut-off value for statistical significance was a p-value < 0.05. Clinical prognostic factors were included in multivariable analysis also with borderline p-values. The R script is available on the GitHub platform.
Results
Sample characteristics
From the beginning of March, there were 47 patients followed up during a 87 days observation period, accounting for a total of 1,035 person days. At the end of follow-up, 28 (60%) patients were discharged, and 19 (40%) died, so the estimated incidence density rate was 0.018 deaths per day (18 SARS-CoV-2-related deaths per 1,000 patient days). Six (13%) patients were admitted to the ICU, and three of them died. Three (6%) patients died within 24 h from admission.
The patients’ median age was 75 (IQR: 59.50–86.00). There were 24 (51%) male patients.
The sample included a couple returning from a journey abroad (4%), 12 (25%) patients reported a history of previous hospitalization, 7 (15%) came from a highly specialized hospital for cardiac surgery, and 15 (32%) were from a residential care home.
The median length of stay was 18 days (IQR: 10.50–34.50), 12 days (IQR: 7–19.50) for non-survivors and 26.50 days (IQR: 11.75–36.25) for survivors.
Comorbidities, symptoms and related therapies, and laboratory findings at hospital admission
The recorded underlying medical conditions included 32 (68%) patients with hypertension, 22 (47%) patients with CVD, 10 (21%) patients with dementia, 9 (19%) were patients affected by type 2 diabetes, 9 (19%) by CKF and 6 (12%) patients with a mental disorder. Four (8%) patients had COPD. Most patients, 33 (70%), presented multimorbidity. There were 5 (11%) patients presenting both diabetes and CVD, patients affected by dementia and CVD numbered 8 (17%), while 2 (4%) patients had all of these three diseases. Among non-survivors, 13 (68%) had hypertension, 11 (58%) had CVD, 7 (37%) had CKF, 7 (37%) had dementia, and 5 (26%) had diabetes (Table I).
Table I
Demographic and clinical characteristics at hospital admission: HRs and 95% CIs from univariate PH Cox model
The most common symptoms were dyspnoea (47%), fever (43%), and cough (34%). Almost all patients were treated with enoxaparin (98%), steroids (96%), and antibiotics (87%), 32 (68%) received hydroxychloroquine and 5 (11%) tocilizumab (Table II). The median PaO2/FIO2 was 259 mm Hg (IQR: 180–380) for the whole sample. It was 207.5 mm Hg (IQR: 137.5–264.2) for non-survivors and 320 mm Hg (IQR: 225–436) for survivors. The median PaO2/FIO2 was 280 (IQR: 230–321) for patients affected by dementia.
Table II
Symptoms and related therapies at hospital admission: HRs and 95% CIs from univariate PH Cox model
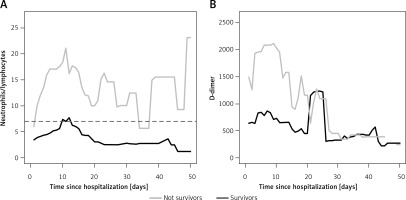
Among the hematologic parameters, the median value for neutrophil/lymphocyte ratio (NLR) was 2.90 (IQR: 1.99–5.57) with lower values for survivors (median = 2.63, IQR: 1.95–4.25) than non-survivors (median = 3.21, IQR: 2.68–8.24). Related blood coagulation, the median value for D-dimer was 509 μg (IQR: 339–1227), and it was higher for non-survivors (median = 1515 μg, IQR: 532–2314) than for survivors (median = 483 μg, IQR: 331–920) (Table III). The day-to-day NLR was on average higher for non-survivors than survivors from the early stage of the disease to discharge/death (Figure 1 A). A similar trend occurred for D-dimer, within the first 20 days of hospitalization (Figure 1 B). The cut-off value for NLR was 7 (Se = 72%; Sp = 83%) and for D-dimer was 855 (Se = 78%; Sp = 63%).
Table III
Laboratory findings at hospital admission and day-to-day: HRs and 95% CIs from univariate PH Cox Model
| Variables | Total sample (n = 47) | Status survivor (n = 28) | Status non-survivor (n = 19) | At hospital admission | Day-to-day | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR§ | 95% CI | P-value | HR§ | 95% CI | P-value | ||||
| Hb | 12.05 (10.95–13.55) | 12.05 (10.28–13.22) | 12.10 (11.32–14.45) | 1.18 | 0.93–1.50 | 0.163 | 0.96 | 0.71–1.29 | 0.772 |
| Eosinophil count [× 10/l] | 10 (0–60) | 15 (6.75–105) | 0 (0–10) | 0.86 | 0.67–1.09 | 0.207 | 0.63 | 0.47–0.83 | 0.001 |
| Lymphocyte count [× 10/l] | 1360 (930–1620) | 1370 (1125–1545) | 1020 (790–1770) | 0.60 | 0.21–1.71 | 0.340 | 0.30 | 0.15–0.58 | < 0.001 |
| Neutrophil count [× 10/l] | 3745 (2718–5858) | 3435 (2652–5155) | 5030 (3305–9008) | 1.99 | 0.84–4.75 | 0.119 | 2.20 | 1.12–4.33 | 0.023 |
| Monocyte count [× 10/l] | 545 (390–772) | 460 (405–780) | 570 (370–770) | 1.05 | 0.63–1.78 | 0.840 | 0.48 | 0.30–0.77 | 0.002 |
| NLR > 7 | 9 (19%) | 3 (11%) | 6 (32%) | 2.52 | 0.90–7.05 | 0.079 | 12.69 | 2.82–57.18 | < 0.001 |
| Platelet count [× 10/l] | 198 (161.5–299) | 219 (163.5–299) | 193 (153.5–268.75) | 0.79 | 0.32–1.92 | 0.599 | 0.88 | 0.67–1.15 | 0.349 |
| Ferritin [μg/l] | 345 (190.2–693.5) | 277 (153.5–590) | 590 (399–1303) | 1.46 | 0.72–2.95 | 0.294 | 0.66 | 0.08–5.36 | 0.701 |
| LDH [mU/ml] | 237 (191.5–291) | 220 (158–262) | 268 (232–760) | 2.90 | 1.29–6.56 | 0.010 | 5.10 | 1.33–19.54 | 0.017 |
| D-dimer [μg] | 509 (339–1227) | 483 (331–920) | 1515 (532–2314) | 2.67 | 1.08–6.60 | 0.034 | 3.43 | 1.60–7.36 | 0.002 |
| C-reactive protein [mg/dl] | 3.38 (1.10–8.95) | 2.45 (0.85–5.84) | 7.13 (2.94–19.39) | 1.07 | 1.03–1.12 | < 0.001 | 1.09 | 1.05–1.13 | < 0.001 |
| Creatinine [mg/dl] | 0.92 (0.67–1.09) | 0.80 (0.65–1.03) | 1.05 (0.83–1.65) | 11.97 | 2.63–54.52 | 0.001 | 2.70 | 1.43–5.09 | 0.002 |
| Bilirubin [mg/dl] | 0.60 (0.40–0.99) | 0.60 (0.50–0.87) | 0.55 (0.37–1.10) | 1.03 | 0.99–1.08 | 0.174 | 0.98 | 0.72–1.35 | 0.927 |
| Troponin [mg/dl] | 7.80 (2.35–14.85) | 5.6 (1.4–12) | 15.65 (12.38–24.10) | 1.12 | 0.77–1.62 | 0.545 | 1.24 | 0.47–3.32 | 0.663 |
| GOT [mU/ml] | 30.5 (20.75–49) | 27.5 (20.25–36.75) | 45 (28.75–58) | 1.01 | 1.00–1.12 | 0.014 | 1.01 | 1.00–1.01 | 0.201 |
| GPT [mU/ml] | 19.0 (11–31.50) | 23 (11.50–29.25) | 19 (11–31.5) | 1.01 | 0.99–1.02 | 0.276 | 0.98 | 0.95–1.01 | 0.176 |
Univariable analysis
There was an increased hazard for age (HR = 1.05, 95% CI: 1.01–1.10), diabetes (HR = 3.48, 95% CI: 1.17–10.37), CKF (HR = 2.87, 95% CI: 1.02–8.05) and cancer disease (HR = 16.23, 95% CI: 2.92–90.08) (Table I). Antibiotic therapy was protective (HR = 0.30, 95% CI: 0.09–0.95) (Table II). Regarding day-to-day laboratory findings, there was a significant hazard for log(LDH) (HR = 5.10, 95% CI: 1.33–19.54), log(D-dimer) (HR = 3.43, 95% CI: 1.60–7.36), C-reactive protein (HR = 1.09, 95% CI: 1.05–1.13) and creatinine (HR = 2.70, 95% CI: 1.43–5.09). Patients with NLR > 7 showed a significantly increased hazard (HR = 12.69, 95% CI: 2.82–57.18) (Table III).
Multivariable analysis
At multivariable analysis, there was a significant hazard for diabetes (HR = 8.13, 95% CI: 1.91–34.67), CKF (HR = 5.86, 95% CI: 1.36–25.21), dementia (HR = 7.84, 95% CI: 1.80–34.20), and NLR > 7 (HR = 10.37, 95% CI: 2.24–48.14) (Table IV). The model’s predictive performance was already satisfactory on the third day of follow-up (AUC ≥ 0.0.905) (Figure 2).
Table IV
Clinical characteristics and day-to-day laboratory findings: HRs and 95%CIs from multivariate PH Cox model
| Variables§ | HR | 95% CI | P-value |
|---|---|---|---|
| Diabetes, Yes | 8.13 | 1.91–34.67 | 0.005 |
| CKF, Yes | 5.86 | 1.36–25.21 | 0.017 |
| Dementia, Yes | 7.84 | 1.80–34.20 | 0.006 |
| NLR > 7, Yes | 10.37 | 2.24–48.14 | 0.003 |
Discussion
In the present study, we described the epidemiological and clinical prognostic factors, therapy and clinical course of SARS-CoV-2 infection in old adult patients, taking full advantage of the longitudinal cohort study design. In this way, we used the day-to-day follow-up to detect some significant prognostic factors of SARS-CoV-2 infection in the province of Palermo at the early stage of the pandemic.
Our sample’s crude fatality rate was 40% (19 out of 47 patients) in a window of time of 87 days. The comparison with other clinical studies is not so immediate, due to heterogeneous study design and patients’ enrolment in the literature. Colaneri et al. [13] estimated a case fatality rate of 4.5% (2 deaths) in a sample of 44 patients enrolled between 21 and 28 February 2020, 57% of whom were over 65. Limiting our observation to a similar time-span, the estimated case fatality rate in our sample in the first week of enrolment was 10.6% (5 deaths), and the percentage of people aged 65+ was 68%. Bruno et al. [5] estimated a case fatality rate of 19% (6 deaths) in a sample of 31 patients enrolled between 25 February and 29 April 2020, all aged 65+. Of note, mortality for SARS-CoV-2 is age-dependent with the highest frequencies observed in young-old (approximately 65–74), middle-old (ages 75–84), and old-old (over age 85) people, the three life-stage subgroups of the older adult population [9].
The median length of hospital stay of survivors in our study was 26 days (Table I), comparable to 23 days reported by Bruno et al. [5] in patients aged 75 years or older admitted to an infectious diseases unit in Southern Italy.
In multivariable analysis, diabetes, dementia and CKF were significantly associated with mortality (Table IV). Diabetes is a known prognostic factor for the progression and mortality for SARS-CoV-2 infection, due to its chronic inflammatory state that causes an imbalance of immune system response. Moreover, SARS-CoV-2 infection is a virus-induced systemic disease characterized by vasculitis and vasculopathy [28], which constitutes a red flag for diabetic patients with COVID-19. Furthermore, the study showed type 2 diabetes mellitus in association with obesity, one of the main prognostic factors for SARS-CoV-2 mortality [29].
Dementia is an emerging prognostic factor for the severe clinical course of the SARS-CoV-2 infection. In our study sample, dementia occurred in 21% of over 65-year-old enrolled patients with a median age of 85.5 years and was associated with severe lung damage, as reported by the analysis of PaO2/FIO2 ratio. The lungs’ imaging features performed during hospitalization supported the severity of pulmonary injury due to SARS-CoV-2 virus infection in dementia patients. Severe acute respiratory syndrome worsens with increasing age and in the presence of pre-existing dementia [30]. We found these patients with an atypical presentation, characterized by a few typical symptoms, specifically dyspnoea, fever and cough. In agreement with this result, Bianchetti et al. [30] found an atypical presentation for dementia patients with SARS-CoV-2 infection.
Prognosis of dementia patients worsens in the presence of both diabetes and hypertension, which are some of the most frequent underlying conditions reported in our study population. Nursing and clinical management of these subjects are challenging, so actions are desirable for implementing clinical knowledge and best practice for the early detection and management of hospitalized dementia patients with SARS-CoV-2 infection.
In our study, there was evidence of increased mortality risk for patients with CKF. Such patients have weaker immune systems, which can explain the increased risk of SARS-CoV-2 mortality by increasing the level of kidney dysfunction [31].
It is well known that NLR is a significant prognostic factor of respiratory and systemic infection [32, 33]. Our study strengthens the role of NLR as a fast, simple and easily measured inflammatory biomarker [33]. Furthermore, we also observed elevated D-dimer levels for non-survivors (Figure 1). The association between increased D-dimers and severe SARS-CoV-2 infection may in part be explained by the interplay between inflammatory response and activation of coagulation. In SARS-CoV-2 patients, D-dimer level is included, especially in the early phase of the disease, as a test to detect severe complications such as deep vein thrombosis or pulmonary embolism (DVT/PE) [34]. The cut-off value of 500 µg/l is intended to exclude PE in otherwise healthy patients [35]. However, the age-adjusted cut-off defined as age × 10 was demonstrated to exclude PE in patients aged 50 years old or older [35, 36]. In our study, the estimated cut-off value for D-dimer was found to be 855, in line with the age-adjusted cut-off. In order to identify severe complications such as acute DVT/PE, D-dimer monitoring is advisable from the first week after symptoms’ onset [37]. Since many SARS-CoV-2 patients showed underlying diseases that may trigger an increase in D-dimer levels, we suggest integrating D-dimer with other parameters such as NLR > 7, the ratio PaO2/FIO2 and the occurrence of other underlying medical conditions, e.g. diabetes, dementia and CKF. We demonstrated the optimal prognostic value represented by the joint clinical evaluation of all these factors already in the first week of follow-up.
The day-to-day follow-up is the main strength of this study, which allowed the ongoing laboratory and clinical data collection, typical of the patient’s initial classification and emergency context. Our study’s main limitation regards the small sample size, which is, however, the total population referred to Partinico COVID Hospital and reflects the extent of the epidemic at the very early stage in the province of Palermo. For best data interpretation, it should be considered that the small sample size can affect the estimates’ precision, as shown by wider confidence intervals. Secondly, missing data in many clinical and laboratory findings prevented us from including them in the statistical analysis.
Despite the evolution of sophisticated adjuncts to healthcare and the improvements of structured critical care systems, the multidisciplinary medical emergency approach and detailed and continuous data collection play a crucial role in detecting significant prognostic factors and improving SARS-CoV-2 infection outcomes in old patients.
The in-depth knowledge of the complete clinical course of SARS-CoV-2 is essential in monitoring and treating the disease in a hospital setting and contributes to improving the patients’ home management, especially older ones with frailty, such as those with dementia and/or diabetes. It has been shown that patients with dementia are at a higher risk of neuropsychiatric disorders due to social isolation and shelter-in-place orders [38, 39] and that SARS-CoV-2 patients affected by diabetes experience deterioration of lifestyle and disruption of their periodic monitoring [40]. Further research may arise from the cooperation with primary health care with the aim to monitor discharged patients and assess the long-term COVID19 impact.