Introduction
The human genome and transcriptome studies have revealed that around 85% of the human genome is transcribed. Nonetheless, it is intriguing that a very low percentage of the transcribed genes code for proteins, indicating that the majority of RNA transcripts are not translated and are therefore non-coding [1]. These non-coding RNA molecules are categorised into microRNAs (around 21 to 24 nucleotides) and long non-coding RNAs (more than 200 nucleotides) [2]. The microRNAs have received much attention and have been extensively studies and many of their cellular and physiological functions have been revealed [3, 4]. Over the last decade or so, studies performed on lncRNAs have revealed their functional association with human diseases such as cancer; for example, aberrant expression of lncRNAs has been shown to be linked to the development of colorectal carcinoma [5], hepatocellular carcinoma [6], breast cancer [7] and glioblastoma [8] to name a few. The lncRNAs have shown involvement in cancer-related processes such as growth, cell death, angiogenesis and metastasis [9]. Papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) is an important lncRNA and was initially shown to suppress the proliferation and metastasis of thyroid cancer via modulation of the S100A4 pathway [10]. Recently, the role of PTCSC3 was also revealed in glioblastoma. It was found that PTCSC3 inhibits the wnt/β-catenin signalling cascade to suppress the growth and invasion of glioblastoma [11]. Nonetheless, the role of PTCSC3 is still largely unknown in other human cancers. This study was therefore designed to investigate the role of PTCSC3 in human oral cancer and attempts were made to explore the underlying molecular mechanisms. Being one of the serious health issues, oral cancer together with pharyngeal cancer is ranked as the 6th most prevalent cancer type across the globe [12]. Annually 0.27 million oral cancer cases are detected throughout the world and most of these cases are reported from the developing countries [13]. The incidence of oral cancer varies geographically with very high frequency in South and Southeast Asia. Generally oral cancer has been shown to be more prevalent in men than in women [14]. The oral cancer risk increases with age and most oral cancers are detected in people above the age of 50 years. However, oral cancer may also be found in younger people. It has been reported that 6% of the oral cancer cases are reported in people below the age of 45 years [15]. The 5-year survival rate for oral cancer is around 5%, which is considered very poor. The patients who survive after successful treatment of oral cancer face some severe consequences of the treatment such as the appearance and function. They have severe issues in eating and speaking, which result in depression and malnutrition [16]. Therefore, there is tremendous urgency to development efficient chemotherapy and potent molecular therapeutic targets for the successful management of oral cancers. In the present study we show that lncRNA PTCSC3 is significantly downregulated in human oral cancer cells and its overexpression causes inhibition of oral cancer proliferation via induction of apoptosis and autophagy.
Material and methods
Tissues, cell lines and transfection of cancer cells
The oral cancer tissues and the normal adjacent tissues were obtained at the Second Affiliated Hospital of Air Force Medical University, Xi’an, China after informed consent from the patients. The cell lines (SCC-4, SCC-9, SCC-15 and SCC-25) along with a normal (EBTr) cell line were procured from ATCC, USA. The culturing of cell lines was performed using Dulbecco’s modified Eagle’s medium (DMEM, Thermo Scientific). The cell lines were maintained in a CO2 incubator at 37°C with 5% CO2 concentration and relative humidity of 98%.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the oral cancer cell lines and tissue line with the assistance of RNeasy kits (Qiagen, Inc., Valencia, CA, USA). To reverse transcribe the cDNA, Omniscript RT (Qiagen, Inc.) was employed using 1 μg of the extracted RNA. The cDNA was then used as a template for RT-qPCR analysis with the assistance of the Taq PCR Master Mix kit (Qiagen, Inc.) according to the manufacturer’s protocol. The cycling conditions were as follows: 95°C for 20 s, followed by 40 cycles of 95°C for 15 s, and 58°C for 1 min. GAPDH was used as an internal control and the relative quantification (2–ΔΔCq ) method was used to evaluate the quantitative variation between the samples.
Transfection
To stably transfect the oral cancer cells with NC and pcDNA-PTCSC3 (GenePharma, Shanghai, China; 10 pmol), Lipofectamine 2000 (Thermo Scientific) was used and the user guidelines were followed.
Cell viability assay
Cell viability of the transfected oral cancer cells was determined by MTT assay. Briefly, transfected SCC-1 and SCC-9 cells were placed in 96-well microplates bearing 100 μl of RPMI 1640 medium with 400,000 cells each well and FBS (5%). Antibiotics were also supplemented to each well plate followed by pre-culturing of both cell lines overnight at 37°C in a CO2 (5%) humidified incubator. Afterwards, MTT stock solution of 50 μl with concentration of 2.5 mg/ml in culture medium was added to each well and subjected to incubation for an additional 1 h. DMSO was used to dissolve the formazan crystal formed from MTT. Finally, the absorbance was determined with an automatic microplate reader (LKB 5060-006 Microplate Reader, Austria) at 540 nm wavelength.
Transmission electron microscopic (TEM) analysis
Autophagy in transfected SCC-1 and SCC-9 cancer cells was investigated via transmission electron microscopy (TEM) analysis. Cells were harvested at 80% growth confluence, and then subjected to trypsinization. Afterwards, cells were first fixed by glutaraldehyde (2%) in phosphate buffer (0.1 M) followed by post fixation with osmium tetroxide (1%). Cells were then exposed to ethanol embedded in resin followed by cutting of thin sections with an ultramicrotome. Finally, these sections were examined on the transmission electron microscope (Zeiss CEM 902) linked to a digital camera for capturing different fields.
Analysis of nuclear morphology
The 4′,6-diamidino-2-phenylindole (DAPI) staining was performed to examine the nuclear morphology of the transfected SCC-1 and SCC-9 cells. Briefly, the cells were cultured in 6-well plates for 24 h at 37°C. About 20 μl of cultured cells were loaded on glass slides for DAPI staining. Finally, slides were covered with coverslips and examined under a fluorescent microscope.
Annexin V/PI assay
To determine the percentage of apoptotic cells, the cultured cells were stained with Annexin VFITC and examined by flow cytometry. For that the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) was used in accordance with provided guidelines. In brief, sub-culturing of transfected SCC-1 and SCC-9 cells at 2.5 × 104 concentration was performed in 6-well plates. Thereafter, both treated cell types were harvested and subjected to PBS washing twice followed by resuspension in 1 × binding buffer. Aliquots of 104 cells were subjected to FITC Annexin V and PI (5 μl each) staining. Finally, a flow cytometer (BD Biosciences, United States) was used to examine each sample.
Transwell cell invasion assay
The transwell chambers containing polycarbonate members with a pore size of 8 μm were put into the six-well plates. The I-type collagen (10 μg/ml) was used for the coating of the lower transwell compartment and subsequently dried. About 200 ml of transfected cells were inoculated in the Matrigel coated upper compartment at the density of 1.5 × 105 cells/ml. The lower compartment was filled with 800 μl of medium containing FBS (20%). The whole system was subjected to incubation at 37°C for 24 h. The cells that invaded through the membrane with Matrigel were stained with crystal violet and photographed under an inverted fluorescence microscope.
Western blotting assay
To examine the protein expressions in transfected SCC-1 and SCC-9 cells, western blotting assay was performed. Briefly, the cells were lysed and lysates were analysed through BCA assay for protein quantification. Around 40 μg of proteins from each sample were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, United States). These membranes were then blotted with primary antibodies of Bax, Bcl-2, LC3-I, LC3-II and Beclin-1 (Santa Cruz, CA, USA) having 1 : 1000 dilution. Thereafter, secondary antibody treatment was performed at 4°C overnight. Finally, an enhanced chemiluminescence reagent (Amersham, Piscataway, NJ, United States) was used for determination of protein bands.
Results
Oral cancer is associated with suppression of lncRNA PTCSC3
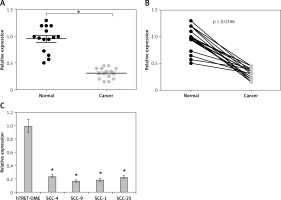
To gain insights about the transcript profile of PTCSC3 in human oral cancer, qRT-PCR was performed. The results showed that PTCSC3 is significantly (p < 0.05) suppressed in oral cancer tissues relative to normal adjacent tissues (Figures 1 A, B). Next, the assessment of the expression profile of PTCSC3 was also done in normal and oral cancer cell lines. The expression of PTCSC3 was found to be significantly (p < 0.05) suppressed in all the oral cancer cell lines relative to the normal cell line. The downregulation was found to be around 5.9-fold. The highest downregulation was found to be lowest in SCC-1 and SCC-9 cell lines. These two cell lines were selected for further experimentation.
Figure 1
LncRNA-PTCSC3 is downregulated in human oral cancer cells. A – Relative expression of PTCSC3 in oral cancer and normal adjacent tissues. B – Pair-wise expression analysis of oral cancer and normal adjacent tissues. C – Relative expression of PTCSC3 in normal and oral cancer cell lines. Individual experiments were performed in triplicate and the results shown as mean ± SD (*p < 0.05)
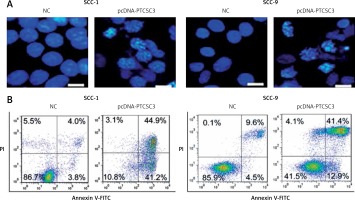
LncRNA PTCSC3 induces apoptosis in oral cancer cells
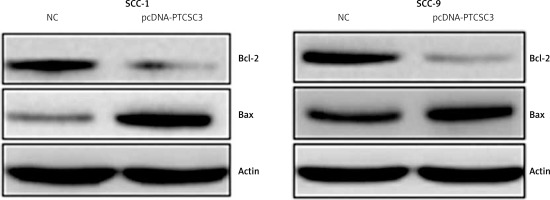
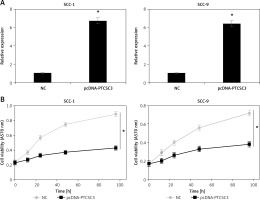
Next, PTCSC3 was overexpressed in SCC-1 and SCC-9 oral cancer cells to gain insights about its functionality. The overexpression of PTCSC3 was authenticated by qRT-PCR analysis; it should about a 6.7- and 6.3-fold increase in PTCSC3 expression in SCC-1 and SCC-9 cells respectively (Figure 2 A). The results of the cell viability assay showed that overexpression of PTCSC3 resulted in a significant decline in the proliferation of the SCC-1 and SCC-9 cells (Figure 2 B). The DAPI staining analysis showed that PTCSC3 overexpression triggers changes in the nuclear morphology of the SCC-1 and SCC-9 cells such as nuclear blebbing and fragmentation indicative of apoptosis (Figure 3 A). The annexin V/PI staining assays showed that the early and late apoptosis increased from 3.8% and 4.0% to 44.9% and 41.2% respectively, upon overexpression of PTCSC3 in SCC-1 cells. In case of SCC-9 cells, early and late apoptosis increased from 4.5% and 9.6% to 12.9% and 41.4% respectively upon PTCSC3 overexpression (Figure 3 B). The expression of Bax and Bcl-2 was evaluated in the NC and pcDNA-PTCSC3 transfected SCC-1 and SCC-9 cells. The results revealed remarkable enhancement of Bax and suppression of Bcl-2 in both the cell lines, further confirming the induction of apoptotic cell death (Figure 4).
Figure 2
PTCSC3 inhibits viability of the oral cancer cells. A – Expression of PTCSC3 in NC and pcDNA-PTCSC3 transfected SCC-1 and SCC-9 oral cancer cells. B – Cell viability of NC and pcDNA-PTCSC3 transfected SCC-1 and SCC-9 oral cancer cells. The experiments were performed in triplicate and the results expressed as mean ± SD (*p < 0.05)
LncRNA PTCSC3 induces apoptosis in oral cancer cells
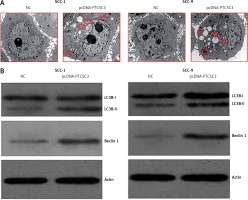
After overexpression of PTCSC3 in SCC-1 and SCC-9 cells, ultrastructural analysis was performed by transmission electron microscopy (TEM). The TEM analysis showed the development of autophagic vesicles in both the SCC-1 and SCC-9 cells indicative of autophagy (Figure 5 A). The western blotting analysis showed that PTCSC3 overexpression caused a remarkable increase in LC3B-I and Beclin 1 expression, further confirming the induction of autophagy in human oral cancer cells (Figure 5 B).
Figure 5
PTCSC3 induces autophagy in oral cancer cells. A – Ultrastructural analysis of NC and pcDNA-PTCSC3 transfected SCC-1 and SCC-9 oral cancer cells. B – LC3B and Beclin 1 expression in NC and pcDNA-PTCSC3 transfected SCC-1 and SCC-9 oral cancer cells. Individual experiments were performed in triplicate
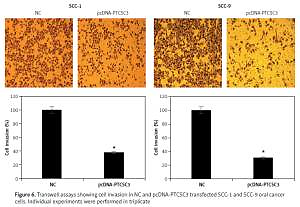
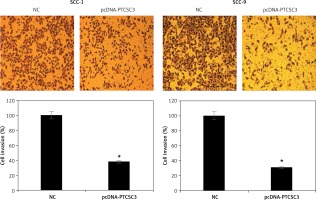
LncRNA PTCSC3 inhibits invasion of oral cancer cells
Transwell assays were performed to assess the effects of PTCSC3 on the invasion of human SCC-1 and SCC-9 oral cancer cells. The results showed that PTCSC3 overexpression caused a significant decrease in invasion of the human SCC-1 and SCC-9 oral cancer cells. Invasion of the SCC-1 and SCC-9 cells was inhibited by 62% and 69% respectively (Figure 6).
Discussion
It is well established that lncRNAs are aberrantly expressed in different cancer types. They play crucial roles in the development and progression of cancers. Studies have shown that lncRNAs may prove essential biomarkers to enable early detection of cancers [17, 18]. Additionally, lncRNAs regulate cancer-related events such as apoptosis, autophagy and metastasis to name a few [19, 20]. As such they may also exhibit therapeutic implications in the management of deadly cancers. This study for the first time examined the therapeutic implications of lncRNA PTCSC3 in human oral cancer. The results showed significant downregulation of PTCSC3 in human oral cancer tissues and cell lines. These findings are in agreement with previous findings wherein PTCSC3 has been shown to be significantly downregulated in human thyroid cancer cells [10]. To decipher the functionality of PTCSC3 in human oral cancer cells, we performed overexpression studies. The results showed that PTCSC3 overexpression caused a significant decline in the proliferation rates of the SCC-1 and SCC-9 oral cancer cells. These observations were consistent with a previous study where PTCSC3 was shown to suppress glioblastoma cell proliferation [11]. Apoptosis and autophagy are essential mechanisms that enable complete elimination of the defective cells. In this study we found that PTCSC3 overexpression triggered both apoptosis and autophagy in human oral cancer cells [21, 22]. This was accompanied by alteration of both apoptosis- and autophagy-related proteins. The apoptosis-related proteins such as Bax were increased while Bcl-2 was decreased. Similarly, autophagy proteins LC3B-II and Beclin 1 also exhibited a remarkable increase upon PTCSC3 overexpression. These findings are in agreement with several other studies wherein lncRNAs have been shown to trigger apoptosis or autophagy in human cancer cells. For example, lncRNA CASC2 overexpression suppressed the proliferation of human hepatocellular cancer and lung cancer cells by triggering apoptotic cell death [23, 24]. In yet another study, lncRNA CCAT2 induced autophagy in human gastric cancer cells [25]. The invasion of the cancer cells from the site of origin to the adjacent tissues is critical for cancer metastasis [26]. In the present study we found that PTCSC3 suppresses the metastasis of human oral cancer. All considered, PTCSC3 may prove an essential therapeutic target for oral cancer treatment.
In conclusion, the findings of the present study show that lncRNA PTCSC3 was significantly suppressed in human oral cancer cells. Overexpression of PTCSC3 resulted in inhibition of the proliferation of human oral cancer cells via induction of apoptosis and autophagy. Additionally, PTCSC3 suppressed the metastasis of human oral cancer cells, indicative of the therapeutic implications of PTCSC3 in oral cancer.