Introduction
Studies have shown that a very small portion of the enormous human genome codes for proteins. The major portion of the human genome does not code for proteins or may be involved in the transcription of non-coding RNAs [1]. Mostly, the non-coding RNAs are categorized as long chain or short non-coding RNAs based on transcript size [2]. During the last several decades, a lot of research has been dedicated to the exploration of the functional roles of microRNAs (miRNAs) owing to their diverse functions [3]. However, in the recent past long non-coding RNAs (lncRNAs), which are generally more than 200 nucleotides in length, have also gained importance due to their roles in a variety of processes [4]. LncRNAs have been shown to be aberrantly expressed in different diseases and are now believed to exhibit the potential to act as therapeutic targets for drugs [5]. Many lncRNAs have been reported to be involved in the development of severe diseases such as cancer. For instance, enhanced expression of lncRNA H19 has been shown to be associated with the metastasis of gastric cancer [6]. Moreover, the lncRNA HOTAIR has been shown to be associated with development of cervical cancer [7]. Some lncRNAs have also been shown to regulate drug resistance in thyroid cancer [8]. LncRNAs have also been implicated in the development of several gynecological diseases [9]. Studies have shown several lncRNAs to be aberrantly expressed in the ovaries of patients with polycystic ovarian syndrome (PCOS) [10]. In a recent study, suppression of lncRNA SRA was reported to attenuate PCOS in mice [11]. Similarly, lncRNA LINC-01572 has been shown to suppress the growth of ovarian granulosa cells of PCOS patients [12]. Moreover, lncRNA SRA has been shown to be associated with the development of PCOS [13]. Given these study findings, it is believed that lncRNAs might act as therapeutic targets for the management of PCOS. PCOS is one of the prevalent endocrine disorders involving hyperandrogenism, insensitivity to insulin and oligo-ovulation [14, 15]. It affects approximately 10% of reproductive women, generally causing infertility [16]. There is concrete evidence that suggests that PCOS increases the risk of type 2 diabetes mellitus [17]. Furthermore, studies have shown that although women exhibiting mild forms of PCOS tend to show normal ovulation, the disorder becomes chronic with time if it remains untreated [18]. PCOS is also associated with increased androgen activity and often interferes with the biosynthesis of estrogen and progesterone [19]. Although insulin sensitizers such as metformin are used for the management of PCOS, such drugs are accompanied by adverse effects that negatively affect the quality of life of patients [20].
Therefore, studies are being directed to identify the molecular mechanisms underlying PCOS to identify the therapeutic targets for the complete treatment of PCOS. The main objective of the present study was to study the role and therapeutic potential of lncRNA H19 in PCOS.
Material and methods
Tissues and cell lines
PCOS ovarian and normal ovarian tissues were obtained from 15 women with PCOS and 15 normal women undergoing laparoscopy or hysterectomy at the Department of Gynecology, Harbin Medical University Cancer Hospital, China. Informed consent was obtained a priori from all the women. All the women were in the same phase of the menstrual cycle and the mean age for normal and PCOS women was 32 years old and 33.5 years old, respectively. PCOS diagnosis was made as described previously [21]. The ovarian granulosa KGN cell line and the normal ovarian cell line SV40 were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The KGN cell line has also been used previously for the study of PCOS [22]. All transfections were carried out using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as per the manufacturer’s protocol. The study was approved by the research ethics board of Yanan University Affiliated Hospital, Yan’an, 716000, China under approval number YU/DO/324 of 2018.
qRT-PCR analysis
Trizol reagent (Invitrogen) was employed to extract RNA from the tissue samples as well as the cell lines. M-MLV reverse transcriptase was used for the synthesis of DNA which was later amplified by SYBR Green master mix (Invitrogen). Expression was assessed by qRT-PCR with actin as an internal control. The cycling conditions were as follows: 95°C for 20 s, followed by 40 cycles of 95°C for 15 s, and 58°C for 1 min.
Cell viability assay
The Cell Counting Kit 8 (CCK-8) was used to examine the viability of the KGN cells. The KGN cells were firstly transfected with appropriate constructs and then cultured for 24 h at 37°C.
About 8 μl of CCK-8 solution was added to the cells and again incubated for 2 h. Finally the absorbance was taken at 450 nm at various time points (0, 12, 24, 48 and 96 h).
Apoptosis assays
Apoptosis was detected by AO/EB staining. The KGN cells were transfected with appropriate constructs and then cultured in six-well plates for 24 h. Around 5–10 μl of culture was taken on the cover slips and stained with AO/EB solution and observed under a microscope for determination of the percentage of apoptosis. The transfected KGN cells were stained with annexin V/PI and subsequently examined by a flow cytometer as described previously [23].
Western blotting
The KGN cells were cultured for 24 h and then collected by the process of centrifugation. Afterwards the cells were lysed by RIPA buffer and the protein content was determined by BCA assay. An equal amount of protein from each sample was loaded on the SDS-PAGE and separated. Thereafter it was transferred to polyvinylidene fluoride membranes which were then treated with TBS and subsequently incubated with primary antibody at 4°C. This was followed by incubation with appropriate secondary antibody, and visualization of the bands of interest was carried out using an enhanced chemiluminescence reagent.
Results
LncRNA H19 was upregulated in granulosa cells of PCOS ovaries
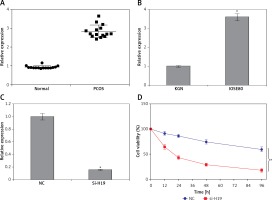
The transcript levels of lncRNA H19 were evaluated in 15 normal and 15 PCOS ovarian tissues. It was revealed that the expression of lncRNA H19 was remarkably increased in the PCOS ovarian tissues. The expression of H19 was around 3-fold higher in PCOS ovarian tissues compared to normal tissues (Figure 1 A). The expression of H19 was also examined in ovarian granulosa-like KGN cells as well as normal SV40 cells. It was found that the expression of lncRNA H19 was increased in the KGN cells by 3.5-fold (Figure 1 B).
Figure 1
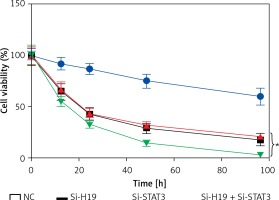
Long non-coding RNA (lncRNA) H19 regulates the viability of polycystic ovarian syndrome (PCOS) ovarian granulosa cells. A – Expression of lncRNA H19 in normal and PCOS ovarian tissues. B – Expression of lncRNA H19 in KGN and normal IOSE80 cells. C – Expression of lncRNA H19 in normal (NC) and Si-H19 transfected KGN cells. D – Cell Counting Kit 8 (CCK-8) assay showing the viability of the NC or Si-H19 transfected KGN cells. The values represent the mean of 3 replicates ± standard deviation (*p < 0.05)
Silencing of H19 inhibited proliferation of KGN cells via apoptosis induction
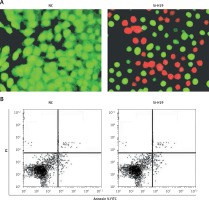
To ascertain the role of H19 in PCOS ovarian granulosa-like KGN cells, the lncRNA H19 expression was suppressed (Figure 1 C). It was revealed that silencing of lncRNA H19 expression in KGN cells resulted in a significant decline in cell viability (Figure 1 D). AO/EB staining showed that suppression of lncRNA H19 led to apoptosis of KGN cells (Figure 2 A). Annexin V/PI revealed that the apoptotic KGN cell percentage increased from 1.77% to 28.15% upon lncRNA H19 silencing in the KGN cells (Figure 2 B). Moreover, lncRNA H19 suppression led to enhancement of Bax and caspase-3, which was also accompanied by a depletion of Bcl-2 (Figure 2 C).
Expression of LncRNA H19 was regulated by STAT3
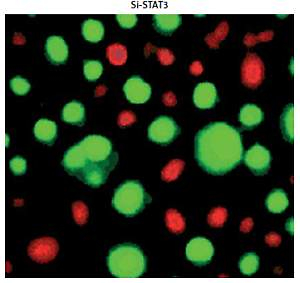
Promoter analysis of the lncRNA H19 by MatInspector software revealed the presence of the GAS binding site for STAT3 (Figure 3 A). Therefore, the expression of STAT3 was suppressed in KGN cells to examine its impact on the expression of lncRNA H19. The suppression of STAT3 in KGN cells was validated by western blot analysis (Figure 3 B). The results showed that silencing of STAT3 in KGN cells also resulted in suppression of lncRNA H19 expression (Figure 3 C). Moreover, silencing of STAT3 expression also caused a decline in the viability of the KGN cells (Figure 4 A). The STAT3 silencing mediated inhibition of KGN cell viability was also found to be due to the induction of apoptotic cell death as evident from the AO/EB and Annexin V/PI staining (Figures 4 B, C).
Figure 3
A – Identification of GAS binding sequence for STAT3 in the promoter of long non-coding RNA (lncRNA) H19. B – Western blots showing expression of STAT3 in normal (NC) and Si-STAT3 transfected KGN cells. C – Expression of lncRNA H19 and STAT3 in NC and Si-STAT3 transfected KGN cells as depicted by qRT-PCR. The values represent the mean of 3 replicates ± standard deviation (*p < 0.05)
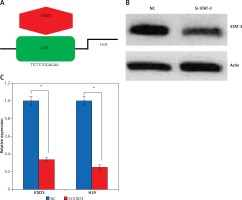
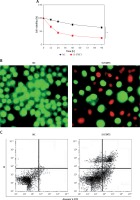
Figure 4
A – Cell Counting Kit 8 (CCK-8) assay showing the viability of the normal (NC) and Si-STAT3 transfected KGN cells. Acridine orange/ethidium bromide (AO/EB) (B) and Annexin V/propidium iodide (PI) staining (C) of the NC and Si-STAT3 transfected KGN cells. The values represent the mean of 3 replicates ± standard deviation (*p < 0.05)
LncRNA H19 and STAT3 acted synergistically in PCOS
To determine whether suppression of both STAT3 and H19 exhibits synergistic effects on the viability of KGN cells, the KGN cells were co-transfected with Si-H19 and Si-STAT3 constructs. It was revealed that growth inhibitory effects of Si-H19 and STAT3 were more significant together then individually, suggestive of synergistic effects (Figure 5).
Discussion
Polycystic ovarian syndrome is one of the prevalent endocrine syndromes in women. It affects 10% of child-bearing women and is one of the causes of infertility in women. LncRNAs have been shown to be associated with the development of several diseases [24, 25]. However, the role of lncRNA H19 has not been explored in PCOS. This study explored the role and therapeutic potential of lncRNA H19 in PCOS for the first time. The expression of H19 was found to be upregulated in PCOS ovarian tissues. These results were in agreement with previous investigations wherein lncRNAs have been reported to be dysregulated in PCOS ovaries [9, 10]. The results of the present study showed that lncRNA H19 regulates proliferation of ovarian granulosa cells. Silencing of lncRNA causes a significant decline in the viability of KGN ovarian granulosa cells via induction of apoptosis. Several studies carried out previously have also indicated the growth inhibitory role of lncRNA H19. It has been reported to cause significant inhibition of the growth of trophoblasts by modulating the expression of DDX3X [26]. LncRNA H19 also interacts with microRNA-140 to suppress the growth of glioma [27]. In yet another study, it was shown to suppress the growth and proliferation of the cervical cancer cells [28]. A previously carried out study indicated an interaction between lncRNA H19 and STAT3 [29]. Therefore, we also analyzed the promoter of lncRNA H19, which revealed the presence of the STAT3 binding site, indicating that STAT3 might be regulating the expression of lncRNA H19. It was further found that suppression of STAT3 caused a significant decline in the expression of lncRNA H19. Moreover, silencing of STAT3 resulted in the apoptotic cell death of KGN cells in a similar way as that of H19 silencing. To ascertain whether STAT3 and H19 have synergistic effects on the growth of KGN cells, Si-H19 and Si-STAT3 were co-transfected into KGN cells, and interestingly, it was found that the effects of lncRNA H19 and STAT3 were more profound when combined than individually. To sum up, this study will reinforce further studies on lncRNAs to explore their potential for the treatment of PCOS.
In conclusion, the findings of the present study revealed that lncRNA-H19 was aberrantly upregulated in PCOS ovarian tissues and granulosa cells, and regulated their growth by interacting with STAT3. Taken together, the evidence suggests that lncRNAs may prove to be a therapeutic target for the management of PCOS and warrants further investigation.