Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
LncRNA NEAT1 contributes to multiple myeloma progression via upregulating ARPC5 by sponging miR-133a
1
Department of Hematology, Hengyang Medical School, University of South China, The First Affiliated Hospital, Hengyang, Hunan Province, China
Submission date: 2023-04-19
Final revision date: 2023-08-30
Acceptance date: 2023-09-11
Online publication date: 2024-03-20
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Long non-coding RNA (lncRNA) nuclear enriched abundant transcript 1 (NEAT1) is confirmed to be involved in regulation of the multiple myeloma (MM) process. However, its underlying molecular mechanism deserves further investigation.
Material and methods:
Quantitative real-time PCR was used to examine NEAT1, microRNA (miR)-133a and actin-related protein 2/3 complex subunit 5 (ARPC5) expression. Cell proliferation and apoptosis were evaluated by cell counting kit 8 assay, soft-agar colony formation assay and flow cytometry. Dual-luciferase reporter assay was used to confirm the interaction between miR-133a and NEAT1 or ARPC5. The localization of NEAT1 and miR-133a in MM cells was determined by fluorescent in situ hybridization assay. In addition, animal experiments were conducted to explore the effect of NEAT1 knockdown on MM tumor growth in vivo.
Results:
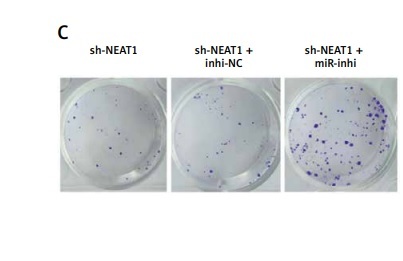
NEAT1 had increased expression in MM patients and cells. NEAT1 knockdown could repress MM cell proliferation and enhance apoptosis. NEAT1 could sponge miR-133a, and miR-133a could target ARPC5. MiR-133a was lowly expressed and ARPC5 was highly expressed in MM patients. MiR-133a inhibitor could abolish the suppressive effect of NEAT1 knockdown on MM cell growth, and these effects also could be reversed by ARPC5 silencing.
Conclusions:
Animal experiments showed that NEAT1 downregulation reduced MM tumor growth by regulating miR-133a/ARPC5 axis. NEAT1 facilitated MM progression through the regulation of miR-133a/ARPC5, which provided new evidence that NEAT1 was a potential therapeutic target for MM.
Long non-coding RNA (lncRNA) nuclear enriched abundant transcript 1 (NEAT1) is confirmed to be involved in regulation of the multiple myeloma (MM) process. However, its underlying molecular mechanism deserves further investigation.
Material and methods:
Quantitative real-time PCR was used to examine NEAT1, microRNA (miR)-133a and actin-related protein 2/3 complex subunit 5 (ARPC5) expression. Cell proliferation and apoptosis were evaluated by cell counting kit 8 assay, soft-agar colony formation assay and flow cytometry. Dual-luciferase reporter assay was used to confirm the interaction between miR-133a and NEAT1 or ARPC5. The localization of NEAT1 and miR-133a in MM cells was determined by fluorescent in situ hybridization assay. In addition, animal experiments were conducted to explore the effect of NEAT1 knockdown on MM tumor growth in vivo.
Results:
NEAT1 had increased expression in MM patients and cells. NEAT1 knockdown could repress MM cell proliferation and enhance apoptosis. NEAT1 could sponge miR-133a, and miR-133a could target ARPC5. MiR-133a was lowly expressed and ARPC5 was highly expressed in MM patients. MiR-133a inhibitor could abolish the suppressive effect of NEAT1 knockdown on MM cell growth, and these effects also could be reversed by ARPC5 silencing.
Conclusions:
Animal experiments showed that NEAT1 downregulation reduced MM tumor growth by regulating miR-133a/ARPC5 axis. NEAT1 facilitated MM progression through the regulation of miR-133a/ARPC5, which provided new evidence that NEAT1 was a potential therapeutic target for MM.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.