Lipoprotein (a) [Lp(a)] is approximately 6-fold more atherogenic compared to low-density lipoprotein (LDL) on a per-particle basis [1]. Strong evidence has shown an independent, causal association between Lp(a) levels and atherosclerotic cardiovascular disease (ASCVD) [2]. Moreover, high Lp(a) levels are a risk factor for aortic valve calcification and stenosis [3]. Recommendations for the diagnosis and management of elevated Lp(a) levels have been recently published by experts from the Polish Cardiac Society and the Polish Lipid Association [4], following the European Atherosclerosis Society consensus statement (2022) [5]. Here, we describe how Lp(a) measurement allowed for personalized lipid-lowering therapy (LLT) in a very high-risk patient with acute myocardial infarction (MI).
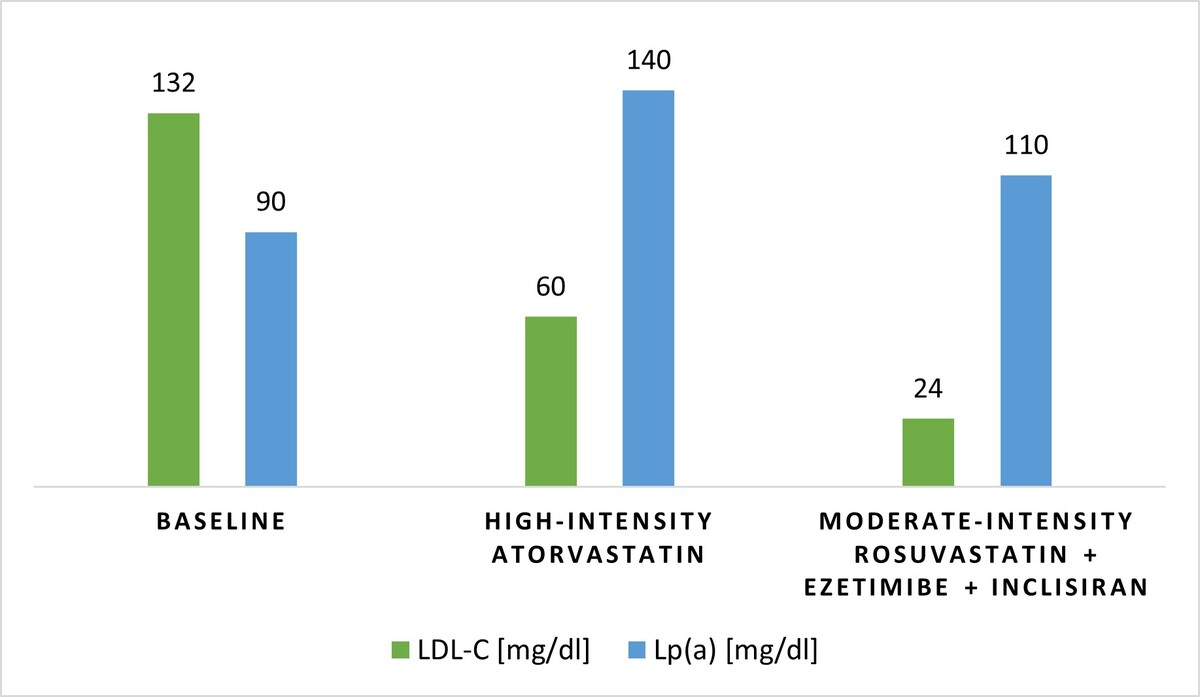
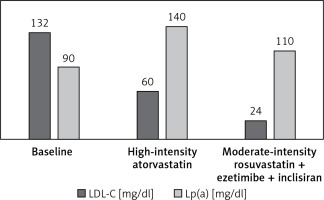
A 58-year-old male triathlete without any prior medical history or medication use was referred to the cardiology department due to deteriorating anginal symptoms. Negative T waves in the inferior electrocardiographic leads (II, III, aVF), along with the elevated concentration of high-sensitivity cardiac troponin T (1564 pg/ml) in the initial measurement confirmed the diagnosis of non-ST-elevation MI. Immediate coronary angiography revealed a total occlusion in the proximal segment of the right coronary artery (RCA), followed by 80% stenosis in the left anterior descending artery (LAD), and 50% stenosis in the circumflex branch. Drug-eluting stents were implanted in the RCA and LAD with good angiographic results. Simultaneously, 12-month dual antiplatelet therapy was initiated. Further diagnostic workup showed mild aortic valve sclerosis with a peak velocity of 1.5 m/s, and bilateral atherosclerotic plaques in the carotid arteries with intima-media thickness of 0.68 mm and 1.04 mm on the left and right side, respectively. Lp(a) measurement was incorporated in the initial lipid profile, and hence, the LDL cholesterol (LDL-C) level of 132 mg/dl (3.41 mmol/l) and Lp(a) level of 90 mg/dl (~225 nmol/l) were found. Other modifiable CVD risk factors were in the optimal range with blood pressure of 132/79 mm Hg, heart rate of 50 beats per minute, body mass index of 21.4 kg/m2, and glycated hemoglobin of 5.4%, accompanied by no history of smoking, and negative family history.
Since the LDL-C target value of this very high-risk patient was < 55 mg/dl (1.4 mmol/l) [6], LLT with high-intensity atorvastatin (40 mg daily) was initiated during the hospitalization. The patient was advised to continue his regular exercises, and along with diet modification towards a plant-based dietary pattern with increased fiber intake, statin treatment resulted in a decrease in the LDL-C level to 60 mg/dl (1.55 mmol/l) at the 8-week control. However, follow-up measurements at a specialized CVD prevention center showed a 56% increase in the Lp(a) level up to 140 mg/dl (~350 nmol/l). A careful screening revealed no risk factors that might have been responsible for this variability. Subsequently, high-intensity statin monotherapy was replaced with a combination of moderate-intensity rosuvastatin (10 mg daily) and ezetimibe (10 mg daily). Moreover, a proprotein convertase subtilisin/kexin type 9 (PCSK9) modulator was added after discussion and shared decision with the patient to intensify the LLT and to reach the guideline-recommended LDL-C level < 55 mg/dl (1.4 mmol/l) [6]. While choosing a PCSK9 modulator, the patient’s preference for infrequent injections was considered, and thus, inclisiran was introduced, resulting in a 60% reduction in the LDL-C level, from 60 mg/dl (1.55 mmol/l) to 24 mg/dl (0.62 mmol/l), and a 21.4% reduction in the Lp(a) level down to 110 mg/dl (~275 nmol/l) at the control visit after 6 months. The LLT course is presented in Figure 1. During 18 months of regular follow-up appointments, no ASCVD progression was recorded and no major adverse cardiovascular events (MACE) occurred. Eventually, given the lack of approved Lp(a)-lowering therapies, the patient was referred to the university hospital and was included in the Olpasiran trials of Cardiovascular Events and Lipoprotein(a) reduction – Outcomes Trial.
Figure 1
The course of lipid-lowering therapy
LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein (a).
Both in the secondary and primary prevention setting, elevated Lp(a) levels are associated with an increased risk of MACE [7]. Thus, according to the recent Polish guidelines, Lp(a) measurement is recommended at least once in the lifetime of every adult and can be considered in very high-risk individuals with ASCVD [4], as in the patient described in this case. Furthermore, the Polish experts indicate the need for repeated Lp(a) measurements in patients with Lp(a) levels between the cut-off values for the risk groups (30–50 mg/dl; 75–125 nmol/l), in patients with kidney disease, or women after the age of 50 years [4]. However, a recent report highlighted a notable intra-individual variability in Lp(a) concentrations among patients with ASCVD and elevated baseline Lp(a) levels. The mean absolute difference observed over 72 weeks was 9 mg/dl (~22.5 nmol/l), whereas the maximum absolute difference reached 54 mg/dl (~134 nmol/l) [8]. Correspondingly, the variation in the Lp(a) level of the described patient was as high as 50 mg/dl (~125 nmol/l). This might partially depend on the type of the assay used at different laboratories, and high-intensity statin therapy [4, 5]. First, variations in the size of the apolipoprotein (a) [apo(a)] isoform make Lp(a) measurement technically challenging. Therefore, an isoform-insensitive assay reporting molar concentrations of Lp(a) is preferred, if available, in the initial and repeated testing [4]. Importantly, a conversion of units from mg/dl to nmol/l is imprecise and not recommended in clinical practice [4, 5]. Second, statins, particularly atorvastatin, might increase Lp(a) levels, especially in patients with a low-molecular-weight apo(a) phenotype [4]. However, an average variation of 6–10% is not clinically relevant and should definitely not lead to statin discontinuation [4, 5].
Considering the management of elevated Lp(a) levels, CVD risk factor optimization comprising lifestyle and drug interventions is recommended in all very high-risk secondary prevention patients [4, 5]. Intensive LLT might be essential in patients with elevated Lp(a) levels, as LDL-C reduction may potentially mitigate the increase in the risk of MACE attributable to high Lp(a) levels [5]. Hereby, early initiation of LLT is crucial, as with a greater lifetime exposure to elevated LDL-C levels, higher LDL-C reduction must be achieved [5]. Nevertheless, Lp(a) remains a CVD risk factor even in statin-treated patients with very low LDL-C levels [9]. Noteworthy, the expert panels suggest considering screening among relatives of individuals with high Lp(a) levels, as these are strongly genetically determined [4, 5]. Therefore, Lp(a) measurement might not only contribute to more accurate stratification of the secondary prevention patient’s CVD risk but might also lead to early identification of those who require comprehensive primary prevention.
Considering LLT regimens for patients with elevated Lp(a) levels, contrary to statins, a mild reduction in Lp(a) levels of approximately 7% was described on ezetimibe treatment [10]. Given its potentially neutral effect, the Polish experts propose that a switch from high-intensity statin monotherapy to a combination of a lower-dose statin and ezetimibe might be considered in high- and very high-risk patients with elevated Lp(a) levels [4]. Interestingly, a recent population-based study found that a combination of a moderate-intensity statin and ezetimibe was associated with a decreased risk of MACE and all-cause mortality, as well as with a higher compliance rate in patients after percutaneous coronary intervention for acute coronary syndrome [11]. Correspondingly, a moderate-intensity statin plus ezetimibe was found non-inferior to high-intensity statin monotherapy for a composite of MACE, non-fatal stroke, and cardiovascular death on a 3-year follow-up in individuals with ASCVD. Moreover, the study patients on combination therapy reached desired LDL-C levels more often than those on monotherapy [12]. These results reinforce the LLT paradigm shift: from high-intensity statin monotherapy to upfront lipid-lowering combination therapy [13].
According to the Polish recommendations, for the management of elevated Lp(a) levels, the addition of a PCSK9 modulator to a high-intensity statin and ezetimibe should be considered an LLT escalation strategy in very high-risk patients, if required to achieve the LDL-C goal. Furthermore, triple therapy may be considered another alternative to high-intensity statin monotherapy, if a combination of a lower-dose statin and ezetimibe is insufficient to reach the LDL-C goal [4]. Although PCSK9 modulators are dedicated to lowering LDL-C levels, Lp(a) reductions ranging from 20% to 30% have been observed in patients receiving monoclonal antibodies, evolocumab, or alirocumab, also with individual response depending on the apo(a) isoform [9]. In addition, inclisiran reduces Lp(a) levels by up to 25% [14]. As the latter is injected only twice yearly, greater adherence may be expected [15]. Overall, if the LDL-C goal cannot be achieved with a statin and ezetimibe, the use of a PCSK9 modulator seems beneficial regarding the concentrations of both LDL-C and Lp(a), and hence, should be considered, particularly when the patient meets the criteria of the drug program, such as the B101 drug program in Poland [4]. However, it should be noted that PCSK9 modulators are not approved for decreasing Lp(a) levels in most European countries. An approach to diagnosis and management of elevated Lp(a) levels, based on the recent Polish recommendations, is summarized in Table I.
Table I
Recommendations for the diagnosis and management of elevated Lp(a) levels. Adapted from [4]
Targeted Lp(a)-lowering therapies are in various stages of development, with three agents, namely pelacarsen, olpasiran, and lepodisiran already being investigated in cardiovascular endpoint trials. For instance, a phase II clinical trial with olpasiran, a small interfering ribonucleic acid inhibiting apo(a) expression, demonstrated significant, dose-dependent mean reductions in Lp(a) levels ranging from 66.9% to 97.5% [16]. Therefore, future management of elevated Lp(a) levels might be based on novel, specific therapeutics [17]. Until then, early, intensive, and personalized LLT, along with comprehensive management of all existing risk factors and concomitant diseases, remains vital. A consultation with a lipidologist or preventive cardiologist might help provide comprehensive, up-to-date treatment. Finally, as the guidelines recommend, Lp(a) measurements for all adults should be advocated among healthcare professionals, along with extensive education [6].