Lipid disorders are prevalent among individuals with heart failure (HF), and among patients with lipid disorders, we also observe individuals with heart failure. However, current evidence suggests that in the absence of other specific indications, such as e.g., cardiovascular disease (CVD), the routine administration of statins in HF patients is not recommended. Nevertheless, it is worth emphasizing that statin therapy does not pose harm to patients who continue its use following the onset of HF [1, 2].
Lipoprotein(a) [Lp(a)], recognized as an independent cardiovascular risk factor for atherosclerotic CVD (ASCVD) [3, 4], has also been investigated in the context of HF risk. Wu et al. demonstrated that in an unadjusted model, a 10 mg/dl increase in baseline Lp(a) levels was associated with an 85% higher risk of developing HF with reduced ejection fraction (HFrEF). Even after adjusting for various covariates, this association remained significant, with a 10 mg/dl increase in baseline Lp(a) correlating with a 17% higher risk of incident HFrEF, reflected by an odds ratio (OR) of 1.17 (95% confidence interval [CI]: 1.05, 1.46) [5]. A meta-analysis by Singh et al. was aimed to clarify the association of Lp(a) and HF. From an initial pool of 360 studies, seven were selected for Mendelian randomization (MR) analysis. The MR meta-analysis revealed that elevated Lp(a) levels significantly increased the HF risk (OR = 1.064, 95% CI: 1.043–1.086, I² = 97.59%, p < 0.001). Leave-one-out sensitivity analysis confirmed the robustness of this association, with ORs ranging from 1.051 to 1.111 [6]. These results highlight the importance of monitoring lipid profiles, including Lp(a) levels, in patients with HF to better understand and manage their cardiovascular risk.
Thus, we aimed to assess the differences between patients with or without HF regarding lipid profile including lipoprotein(a) as well as comorbidities and pharmacotherapy.
Methods
We included 510 consecutive patients from the departments of cardiology and endocrinology, as well as outpatient metabolic and lipid disorder clinics – as a part of the PMMHRI-Lp(a) Registry (NCT06610669), which design and details were presented elsewhere [7]. Medical history was retrospectively extracted from the electronic health records (EHRs). For the purposes of our study, we collected data regarding lipid profile with Lp(a), demographic data, comorbidities, and concomitant medication. We ranged Lp(a) levels based on the following categories: < 30 mg/dl (< 75 nmol/l), 30–50 mg/dl (75–125 nmol/l), > 50–180 mg/dl (> 125–450 nmol/l) and > 180 mg/dl (> 450 nmol/l). Statistical analysis was conducted utilizing Statistica v.13 software (TIBCO Software Inc., Palo Alto, CA, USA). Continuous variables were expressed as mean ± SD or median (25th–75th percentile), while categorical variables were presented as proportions. Group comparisons employed Student’s t-test for independent variables and the Mann-Whitney U test or χ2 test with Yates’s correction, as applicable. A significance level of p < 0.05 was adopted for all calculations to denote statistical significance. Analysis of the relationship between dependent and independent variables was performed with the use of logistic regression with a backward stepwise method.
Results
Among our population HF was diagnosed in 59 (11.57%) patients. Patients with HF (mean: 65 ±12, median: 66 (58–73)) were significantly older compared to those without (mean: 46 ±21, median: 50 (33–62)) (p < 0.001). No significant differences were observed in weight, height, systolic and diastolic blood pressure, and heart rate between the two groups (Table I). In patients with HF the following diseases were more prevalent: chronic coronary syndromes (CCS) (67.8% vs. 9.53%, p < 0.001), myocardial infarction (50.85% vs. 3.99%, p < 0.001), stroke (10.17% vs. 3.10%, p = 0.009), hypertension (83.05% vs. 37.11%, p < 0.001), diabetes mellitus (33.90% vs. 18.89%, p = 0.007), left bundle branch block (6.78% vs. 0.89%, p = 0.001), arrhythmias (31.58% vs. 9.82%, p < 0.001), and chronic kidney disease (18.64% vs. 2.22%, p < 0.001). Former and current smokers were significantly more prevalent in the HF group (21.05% vs. 9.45% and 40.35% vs. 7.21%, respectively; all p-values < 0.001), while never smokers were more common in the non-HF group (38.60% vs. 83.33%) (Table I).
Table I
Comparison between patients with and without heart failure
[i] BMI – body mass index, BP – blood pressure, TC – total cholesterol, LDL-C – low density lipoprotein cholesterol, HDL-C – high density lipoprotein cholesterol, TG – triglyceride, Lp(a) – lipoprotein(a), ALT – alanine aminotransferase, AST – aspartate aminotransferase, eGFR – estimated glomerular filtration rate, WBC – white blood cells, RBC – red blood cells, HGB – hemoglobin, PLT – platelet counts, TSH – thyroid stimulating hormone, HbA1c – hemoglobin A1c, LBBB – left bundle branch block, PCSK9i – proprotein convertase subtilisin/kexin 9 inhibitors – ACEI – angiotensin-converting enzyme inhibitors, CCB – calcium channel blockers.
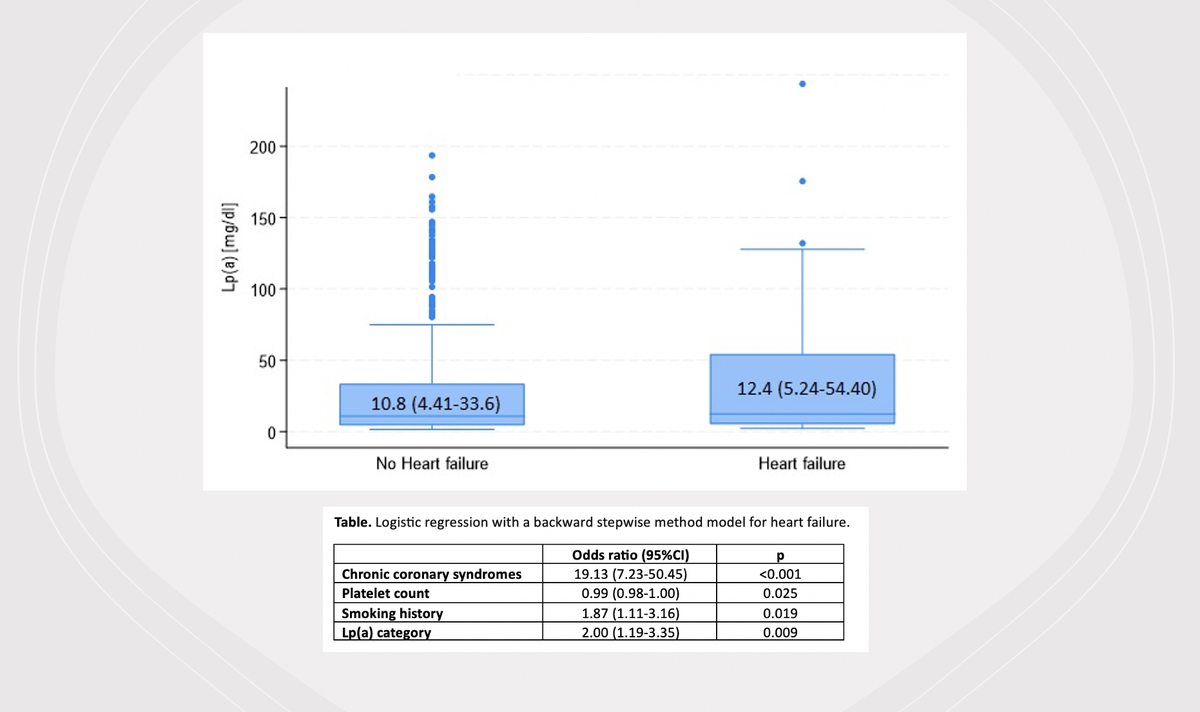
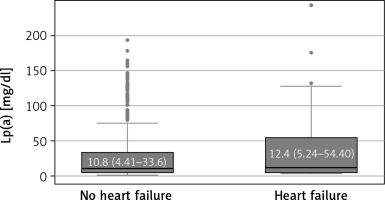
Significant differences were also observed in various blood parameters: patients with HF had lower red blood cell count (p = 0.002), hemoglobin (HGB) (p = 0.042), platelet count (PLT) (p < 0.001) and estimated glomerular filtration rate (eGFR). Patients with HF had higher levels of urea (p = 0.012), creatinine (p < 0.001), indicative of impaired kidney function. Among lipid profile parameters, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), non-HDL cholesterol, were significantly lower in the HF group (p < 0.001; p < 0.001; p = 0.006 and p < 0.001, respectively), while there were no statistically significant differences regarding triglycerides (TG) (Table I). Higher levels of lipoprotein(a) [Lp(a)] were observed in the HF group, but it was not statistically significant (Figure 1). There was also no statistically significant association between Lp(a) categories and HF. The correlation analysis between Lp(a) and coronary artery disease (CAD) revealed a very weak positive correlation (r = 0.0253). This suggests that there was a minimal linear relationship between Lp(a) levels and the presence of CAD in this dataset. The applied pharmacotherapy, including statins, ezetimibe (as a part of lipid lowering combination therapy), proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, anticoagulants, diuretics, calcium channel blockers were significantly more prevalent in the HF group (Table I).
The multivariable analysis using logistic regression with a backward stepwise method revealed four significant predictors of HF, including CCS (OR = 19.13, 95% CI: 7.23–50.45, p < 0.001), PLT, which was inversely associated with HF risk (OR = 0.99, 95% CI: 0.98–1.00, p = 0.025), history of smoking (OR = 1.87, 95% CI: 1.11–3.16, p = 0.019), and higher category of Lp(a) level, which was significantly associated with a higher HF risk (OR = 2.00, 95% CI: 1.19–3.35, p = 0.009). The alternative model that included Lp(a) level as a continuous variable, instead of categorization of Lp(a), showed an even higher level of significance.
Discussion
While hypercholesterolemia is a significant risk factor for coronary artery disease (CAD) [8], we may assume that among patients with lipid disturbances there will be those with HF, a prevalent and severe medical condition frequently resulting from CAD. However, it is paradoxical that individuals with advanced HF may exhibit low cholesterol levels, which correlates with a worse prognosis [9]. Familial hypercholesterolemia (FH) and elevated non-fasting triglycerides have been linked to an increased risk of HF [10]. Conversely, low HDL and LDL-C levels might be strongly correlated with poor outcomes in severe or end-stage HF, regardless of the underlying cause (however in some analysis it might be a reverse causality) [11]. Additionally, low serum TC levels are independently associated with poor prognosis in end-stage HF patients, increasing mortality in both ischemic and non-ischemic HF [12]. In our analysis of lipid profile parameters, TC, HDL-C, and non-HDL-C were significantly reduced in the HF group, which is consistent with the previously described HF lipid-paradox [9]. In contrast to this, no statistically significant differences were observed for LDL-C and TG levels. However, none of the traditional lipid profile parameters was statistically significant in the multivariable analysis. On the other hand, we observe only numerical differences in Lp(a) levels in patients with or without HF (higher in the former group) and showed that a higher category of the Lp(a) level was strongly associated with a higher HF risk in the multivariable analysis. Other studies also showed the role of elevated Lp(a) in HF. Yan et al. conducted an observational study with 309 patients, of whom 10.03% were lost to follow-up during a median follow-up period of 186 days. Multivariate Cox regression analysis confirmed that elevated Lp(a) levels independently predicted recurrent HF, even after adjusting for confounders (HR = 2.720, 95% CI: 1.730–4.277, p < 0.0001). Kaplan-Meier analysis showed that higher Lp(a) levels were associated with a greater incidence of recurrent HF (log-rank p < 0.0001) [13]. Another prospective observational study which recruited 362 HFrEF patients showed that elevated Lp(a) levels remained independently associated with higher cardiovascular mortality (HR = 1.22, 95% CI: 1.04–1.64, p = 0.02) and composite endpoint (HR = 1.38, 95% CI: 1.16–2.01, p = 0.006) after adjusting for covariates [14]. The above-mentioned meta-analysis of Singh et al. also showed that elevated Lp(a) levels significantly increased the risk of HF (OR = 1.064, 95% CI: 1.043–1.086, p < 0.001). The underlying mechanisms, whether they are increased inflammation and thrombotic risk, direct effects on heart muscle contraction or increased risk of ischemic cardiac disease, still require further investigation [6]. Another explanation for the link between Lp(a) and HF is significantly influenced by its impact on CAD and calcific aortic valve stenosis (CAVS). A large case-control study with 143,087 participants confirmed that Lp(a) genetic variants are associated with HF risk. Specifically, each 50 nmol/l (~20 mg/dl) increase in Lp(a) concentration corresponded to a 5% higher risk of developing HF [15].
In conclusion, HF patients typically exhibit advanced age, modified blood parameters indicative of possible renal and metabolic dysfunction, and unfavorable lipid profiles [16, 17]. The level or category of Lp(a) may play a more significant role than other lipid parameters in HF patients, warranting further investigation and emphasizing the need to measure Lp(a) in these patients. Our findings underscore the multifactorial nature of HF and highlight the importance of managing CCS, platelet count, smoking habits, and Lp(a) levels in mitigating HF risk.