Introduction
Numerous studies have demonstrated that elevated lipoprotein(a) [Lp(a)] is an independent risk factor for coronary heart disease (CHD), ischemic stroke, and calcific aortic valve stenosis (CAVS) [1–4]. Despite extensive evidence linking elevated Lp(a) levels to cardiovascular disease (CVD), to date, no approved pharmacologic therapies directly target Lp(a). Currently, clinical trials are underway on drugs that can lower Lp(A) levels [5–7], so with the development of therapies effective at lowering Lp(a), it is essential to understand the prevalence and patterns of elevated Lp(a) levels in the very high-risk population.
Lp(a) is a circulating low-density lipoprotein (LDL-C) in which a large glycoprotein, apolipoprotein(a) [Apo(a)], is bound via a disulfide bridge to apo B100 [4]. It comprises a low-density lipoprotein-like moiety covalently linked to a genetically mediated apolipoprotein(a) molecule. It is more atherogenic than LDL because the additional apolipoprotein(a) component may exacerbate atherothrombosis by promoting vascular inflammation, and its potential antifibrinolytic activity is associated with inhibition of plasminogen [8, 9]. An Lp(a) level above 30 mg/dl (≈75 nmol/l) is the threshold at which its impact on atherosclerotic cardiovascular disease (ASCVD) has been shown to become clinically meaningful [8].
Data on the Lp(a) levels regarding laboratory and clinical effects are still very scarce in Poland. Large national registries containing Lp(a) level information are not available. Therefore, we decided to evaluate the demographic data, clinical characteristics, and outcomes of a large cohort of very high-risk patients hospitalized in a tertiary, superregional hospital to investigate the prevalence of this risk factor and further understand the potential relationship between Lp(a) levels and atherosclerotic cardiovascular events.
Thus, the main aims of the Zabrze-Lipoprotein(a) Registry (Zabrze-Lip(a)R) are to (1) assess the concentration of Lp(a) in the population of very high-risk patients in Poland; (2) determine the association of Lp(a) level and the age of ASCVD events; (3) determine the association between Lp(a) level and progression of aortic stenosis and/or need for aortic valve replacement; (4) assess the correlation of Lp(a) concentration with the occurrence of cardiovascular events during long-term follow-up. These results may significantly improve our knowledge in this field and help us plan and introduce suitable actions for both clinical practice and the healthcare system.
Material and methods
Study design and participants
The Zabrze-Lip(a)R was designed to determine Lp(a) levels in very high-risk patients. All consecutive, very high-risk patients admitted to the Third Clinic of Cardiology, Silesian Center for Heart Diseases (tertiary, superregional hospital) University Hospital in Zabrze, Poland, have been included in the registry. The ESC/EAS 2019 definition of very high-risk patients was adopted [10]. All individuals ≥ 18 years of age with at least 1 Lp(a) result were included in our cohort.
Serum Lp(a) was determined using immunoturbidimetry (Siemens Healthineers, Erlangen, Germany). In order to validate the measurement method, two measurements of Lp(a) concentration were performed in a group of 300 patients, showing high compliance with the measurements (98%). We used the cut-off point of Lp(a) levels of < 30 mg/dl (< 75 nmol/l) for normal values; 30–50 mg/dl (75–125 nmol/l) for moderate risk, > 50–180 mg/dl (> 125–450 nmol/l) for high risk and > 180 mg/dl (> 450 nmol/l) for very high risk based on the Polish Lipid Guidelines (2021) [11] and the PCS/PoLA recommendations on Lp(a) management (2024) [4]. Due to the limitations of laboratory methods, it was impossible to present the detailed results of patients with Lp(a) levels > 75 mg/dl (187.5 nmol/l).
After being discharged, the patients will remain under ambulatory care. The following information within at least a 12-month observation period will be gathered from the data of the National Health Fund (NFZ) in Poland:
The death date (caused either by cardiac or noncardiac events),
Nonfatal myocardial infarction (MI),
Planned or ACS-caused revascularization.
This study was conducted in accordance with the World Medical Association’s Declaration of Helsinki and informed written consent was obtained from all participants. The Ethics Committee of the Medical University of Silesia approved this study involving human participants.
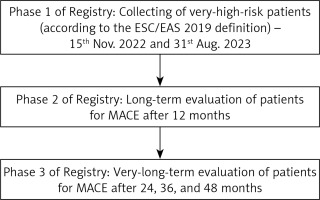
The study flowchart is shown in Figure 1.
Definitions
Chronic coronary syndrome (CCS) was diagnosed in patients according to the guidelines as a group of clinical symptoms caused by myocardial ischemia, which is a result of atherosclerotic coronary artery occlusion. The disease presents as chest pain caused by physical activity or stress, which subsides after rest or taking nitroglycerin [12].
Acute coronary syndrome (ACS) was diagnosed in compliance with the guidelines in cases where, along with typical symptoms presented by patients, the following syndromes were observed:
– preserved (> 20 min) ST-segment elevation in specific adjacent ECG leads – ST-elevation myocardial infarction (STEMI);
– non-preserved ST-segment elevation in ECG but with increasing myocardial ischemia marker levels in laboratory tests (non-ST-elevated myocardial infarction – NSTEMI);
– non-preserved ST-segment elevation in ECG and no increasing myocardial ischemia marker levels in laboratory tests – unstable angina (UA) [12].
Hypercholesterolemia was defined as increased fasting total plasma cholesterol levels exceeding 200 mg/dl (5.2 mmol/l), hypertriglyceridemia as triglyceride plasma level higher than 150 mg/dl (1.7 mmol/l), and combined hyperlipidemia was defined as increased cholesterol and triglyceride levels, when not treated with statins [13]. Furthermore, patients who on admission had been treated with statins for hypercholesterolemia were classified as hypercholesterolemic despite their normal cholesterol levels measured on admission. Lipid level measurements (total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG)) were performed at least once on every patient on the day of admission. Target levels of all lipid fractions in pharmacologically treated patients were set in line with the European and national guidelines [10, 13]. Treatment targets were based on the valid guidelines as LDL-C < 55 mg/dl (< 1.4 mmol/l) for the patients with very high cardiovascular risk.
Diabetes mellitus was ascertained when it had been diagnosed during outpatient visits before admission or when the patient’s fasting glycemia exceeded 126 mg/dl twice or was higher than 200 mg/dl in a random glucose test or a 2-hour oral glucose tolerance test (OGTT) [14]. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2 [9]. Arterial hypertension was defined as previously diagnosed and treated hypertension or when arterial blood pressure (BP) values were higher than 140/90 mm Hg [15].
Chronic heart failure (CHF) was defined based on the occurrence of heart failure symptoms, decreased left ventricular ejection fraction (LVEF), or diastolic function disorders, and/or when the N-terminal prohormone of brain natriuretic peptide (NT-proBNP) marker level exceeded 125 pg/ml [15].
A positive family history of CAD was defined as an incidence of myocardial infarction or chronic coronary syndrome in a first-degree relative [13].
Nicotinism was diagnosed in the case of regular tobacco smoking 1 year before admission. The patients who had not been smoking for at least a year before admission were classified as former smokers [15].
Statistical analysis
All analyses were performed on anonymous data. Basic parameters of descriptive statistics for the analyzed continuous variables were presented as the mean and standard deviation (SD) for normal distributions or as the median and quartiles 1 and 4 (Q1–Q4) for non-normal distributions. Qualitative variables were presented as numeric and percentage values. The normality of distribution was verified using the Shapiro-Wilk test. The comparison between groups regarding continuous variables was tested using the Mann-Whitney U test due to non-normal distribution of compared variables. A two-sided p-value less than 0.05 was considered significant. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) was used for all calculations.
In the follow-up period, the clinical characteristics of the patients will be compared across different subgroups, including sex, risk factors and concomitant disorders. Continuous variables will be reported as means/medians and compared with t-tests, rank-sum tests, or analysis of variance, as appropriate. Categorical variables will be notified as frequencies and proportions and will be compared with χ2 or Fisher exact tests. Analyses will be adjusted using regression techniques for available baseline variables to adjust for confounding and to examine associations between Lp(a) level and the outcomes of interest (primary and secondary). Cox proportional hazards modeling will also be performed for time-to-event analyses. Incidence rate ratios will be calculated when comparing the rates of CVD events between different levels of Lp(a).
Results
The registry finally included 2001 consecutive very high-risk cardiovascular patients, according to the ESC definition [10], admitted to the clinic during the period between 15th November 2022 and 31st August 2023. The mean age of patients was 66.4 (58.9–76.1) years. Female patients represented 37.1% of the population. 312 (15.6%) patients were admitted with acute coronary syndrome (ACS), 889 (44.4%) with chronic coronary syndrome (CCS), 814 (40.7%) patients were after previous coronary angioplasty, and 142 (7.1%) after CABG. The baseline characteristics of the entire cohort are represented in Table I.
Table I
Zabrze-Lip(a)R: Characteristics of the group (N = 2001 patients)
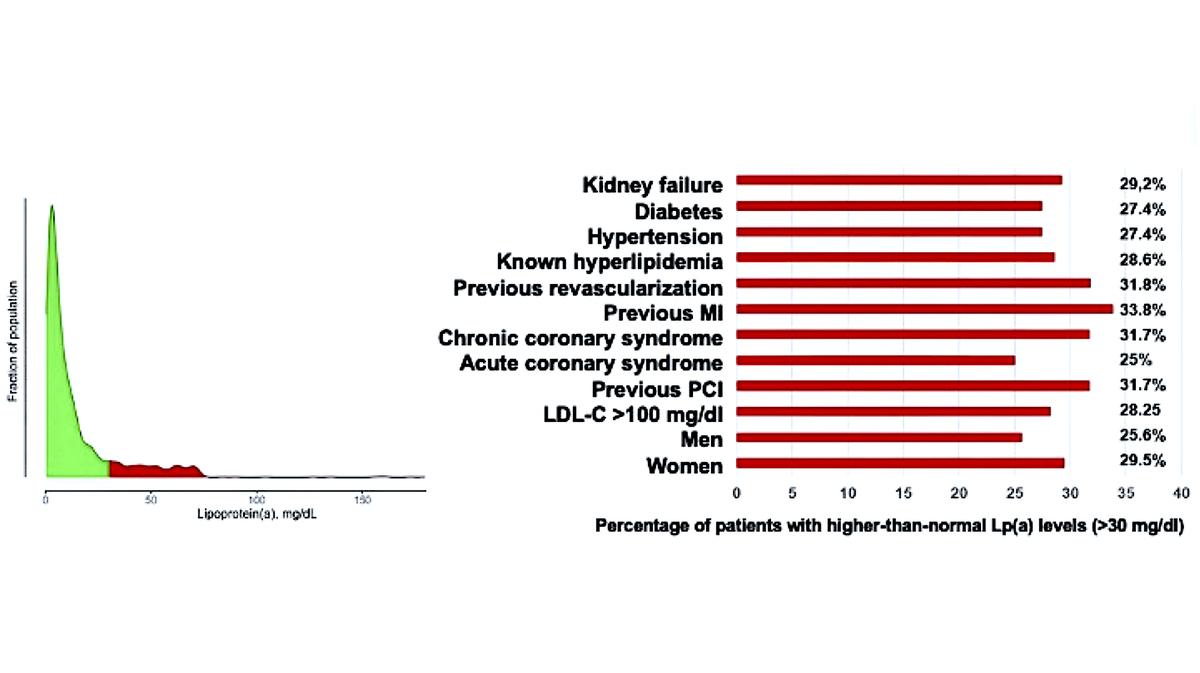
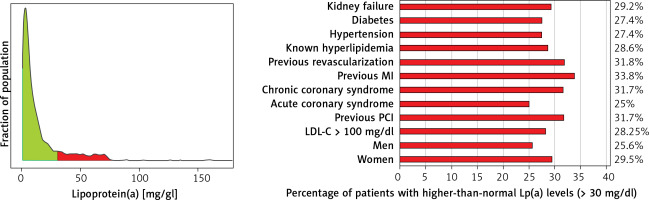
The median Lp(a) concentration in the entire population was 6.6 mg/dl (16.5 nmol/l) (mean 14.3 ±19.4 mg/dl). 540 (27%) patients had elevated Lp(a) levels above 30 mg/dl (75 nmol/l), mainly in patients with established ASCVD after MI and/or coronary revascularization; Lp(a) > 50 mg/dl (125 nmol/l) was reported in 418 (20%) patients and > 75 mg/dl (187 nmol/l) only 58 (2.9%) patients. Patients with elevated Lp(a) > 30 mg/dl were significantly older than patients with normal Lp(a) concentration (66.3 vs. 68.8 years, p = 0.04). Elevated Lp(a) concentrations > 30 mg/dl were found significantly more often in patients with CCS (52.2% vs. 41.5%; p < 0.001), in patients undergoing PCI during hospitalization (23.9 vs. 19%; p = 0.01), and in patients with previous MI (20.6% vs. 14.9%; p = 0.002). Interestingly, in patients admitted to the hospital with ACS there were significantly more patients with elevated concentration of Lp(a) > 30 mg/dl (75 nmol/l) (14.6% vs. 18.3%; p = 0.04) (Table II).
Table II
Comparison of patients with normal and elevated Lp(a) levels – clinical data
[i] EF – ejection fraction, BMI – body mass index, ACS – acute coronary syndrome, CCS – chronic coronary syndrome, CHF – congestive heart failure, MI – myocardial infarction, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, AF – atrial fibrillation, TIA – transient ischemic attack.
This group of patients also had significantly lower hemoglobin, and higher hematocrit, platelet counts and levels of NT-proBNP and CRP (16.1 vs. 9.9 mg/l, p = 0.01). No significant differences were found between the groups regarding BMI, LVEF, TC, LDL-C, or TG levels (Tables III). Regarding the percentage of patients with elevated Lp(a) levels in relation to the whole cohort, a higher percentage was observed in patients with previous MI (33.8%), after previous revascularization (31.8%), and with chronic coronary syndrome (31.7%) (Table IV, Figure 2).
Table III
Comparison of patients with normal and elevated Lp(a) levels – laboratory data
[i] Hb – hemoglobin, HCT – hematocrit, PLT – platelet count, TC – total cholesterol, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, TG – triglyceride, e-GFR – estimated glomerular filtration rate, NT-proBNP – N-terminal pro-B-type natriuretic peptide, AspAT – aspartate aminotransferase, AlAT – alanine aminotransferase, CRP – C-reactive protein.
Table IV
Percentage of patients with higher-than-normal Lp(a) levels (> 30 mg/dl)
In the multivariable analysis, the independent predictors of elevated Lp(a) above 30 mg/dl were only lower Hb values, with an odds ratio (OR) of 0.925 (95% confidence interval (CI) 0.874–0.978; p = 0.006), and higher platelet count (PLT), OR = 1.002 (95% CI: 1.000–1.003; p = 0.0196).
Discussion
To our best knowledge these are the first results on the Lp(a) levels in ASCVD patients in Poland. We found that every 4th (27%) high right patient at very high CVD risk has moderately elevated Lp(a) levels, and every 5th (20%) such patient has highly elevated levels > 50 mg/dl (125 nmol/l). The prevalence of increased levels of Lp(a) > 30 mg/dl (75 nmol/l) are the highest (every 3rd patient admitted to hospital) in those with a history of previous MI, previous revascularization and with CCS. The multivariable analysis revealed low Hb and high PLT as independent risk factors of increased Lp(a) above 30 mg/dl (75 nmol/l), which again might raise a discussion on the role of Lp(a) as a prothrombotic factor.
Although Lp(a) was discovered 61 years ago, interest in this atherosclerosis risk factor has only recently increased. Lp(a) concentration is known to be 90% genetically determined, but its physiological role in humans still needs to be fully explained [16, 17]. The results of previous work indicate that physiologically Lp(a) accelerates wound healing and tissue repair [18, 19]. It has been noted that Lp(a) accumulates in endothelial lesions, where it binds to components of the vessel wall and subendothelial matrix, stimulates chemotactic activation of monocytes/macrophages, and modulates angiogenesis [20]. These effects highlight the function of Lp(a) as a potent modulator of tissue remodeling, but the exact mechanisms also contribute to the persistent development of atherosclerotic plaque. Studies on animal models additionally suggest that apo(a) fragments formed as a result of proteolytic degradation of Lp(a) might be responsible for the antiangiogenic and anticancer effects [20]. Interestingly, people with extremely low plasma Lp(a) concentrations do not develop any diseases or deficiency syndromes [18].
The role of Lp(a) in the development of atherosclerosis has been known since the 1970s. However, the lack of effective drugs lowering its concentration resulted in little interest in this lipoprotein. Similar to other lipoproteins, Lp(a) is susceptible to oxidative modifications, leading to the formation of pro-inflammatory and pro-atherogenic oxidized phospholipids, oxysterols, and oxidized lipid-protein adducts in Lp(a) particles, leading to atherosclerotic lesion progression [20]. Drugs available on the market that lower LDL-C levels (statins, ezetimibe, fibrates) do not reduce Lp(a) levels [21]. Lp(a) apheresis has remained the only effective treatment available [11]. Recently, the appearance of new drugs (PCKS9 protein inhibitors, inclisiran) and drugs selectively lowering Lp(a) concentration (e.g., pelacarsen, olpasiran, lepodisiran, zerlasiran, muvalaplin) has increased interest in this molecule [4, 22].
Our registry includes 2,001 patients with very high cardiovascular risk. It is designed with a long-term follow-up (at least 5 years) to assess the incidence of MACE (deaths, MI, and stroke). In this respect, it is similar to the registry of the Danish Copenhagen City Heart Study [2], which assessed the incidence of MI in a population with extremely high Lp(a) levels. Our registry is also similar in design to the Bruneck Study [23], with the difference that the Bruneck Study assessed the occurrence of MACE in a population of 1,000 healthy residents of Bruneck and not in hospitalized patients with very high cardiovascular risk. The Emerging Risk Factors Collaboration study combined data from 36 studies and analyzed a group of over 125,000 patients regarding the effect of Lp(a) on incidence of MACE [24]. Other data on Lp(a) concentrations most often come from different studies and were obtained “on the occasion” of the main study, e.g., from the Women’s Health Study (WHS) [25], the UK Biobank database [26, 27], or from the EPIC-Norfolk Prospective Population Study, which included 25,663 men and women aged between 45 and 79 years living in Norfolk, UK [28]. In turn, one of the largest registries dedicated to Lp(a) is the Mass General Brigham Lp(a) Registry, which collects data from approximately 30,000 patients in whom Lp(a) concentration was determined as part of routine clinical care. Unfortunately, this registry is limited by the fact that it is retrospective and includes data from the period from January 2000 to January 2019 only [29]. Hopefully a similar registry including even more patients will soon be established as a national project of the Polish Lipid Association (PoLA).
So far, there have been no published results of studies on Lp(a) concentrations in large groups of patients, either healthy – from the general population – or in selected groups, such as our registry, which would cover the region of Central and Eastern Europe, and in particular Poland. Data on MACE in our registry will be collected prospectively in the coming years of observation, but even the first results from our population show some interesting facts worth describing. In our registry, only 27% of patients have Lp(a) levels higher than 30 mg/dl (75 nmol/l). In contrast, in the cross-sectional, epidemiological, multicenter study conducted in 48 countries by Nissen et al., Lp(a) levels above 30 mg/dl were found in 38.4% of patients. However, this group had a higher risk of cardiovascular events, such as MI (72.9%), stroke (12.5%), or PAD (9.2%) [30].
Secondly, in the Lip(a)R registry, compared to, e.g., the EPIC-Norfolk registry, the patients are older (66.5 vs. 59.2 years), but the average TC and LDL-C concentrations are lower: 163 mg/dl (4.3 mmol/l) vs. 236 mg/dl (6.2 mmol/l) and 91 mg/dl (2.4 mmol/l) vs. 152 mg/dl (4.0 mmol/l), respectively. This is most likely due to the fact that patients with very high cardiovascular risk in our cohort initially receive lipid-lowering treatments [31]. Thirdly, due to the specific nature of the group of patients with very high cardiovascular risk, we have a higher number of patients with atherosclerosis risk factors in the registry. 70% of patients have hypertension and 29% have diabetes. Almost half of them were patients after revascularization, 16% of patients had MI, and approximately 16% had previously been diagnosed with heart failure. Moreover, the mean LVEF is 45.2 ±13.2%, which also indicates previous myocardial damage. To the best of our knowledge, no previous registries have assessed Lp(a) levels only in patients with recent MI. In the EPIC-Norfolk group, only 3.2% of patients had MI, and 3% had diabetes. In the UK-Biobank group, 29.6% had hypertension and 5.5% diabetes [28, 32]. Fourthly, no relationship between Lp(a) and CRP was found in other registries, and such a relationship may indicate the pro-inflammatory effect of this molecule. Fifthly, the highly statistically significant correlation between lower hematocrit and higher Lp(a) concentration is interesting. This relationship requires further research in subsequent years of observation of the study group to confirm whether these results may have any clinical relevance and to indicate whether Lp(a) is a prothrombotic risk factor that requires therapeutic intervention (aspirin, which is still debatable due to inconsistent results) [32]. A recently published Polish registry of 800 healthy people aged 40–65 from the Małopolska Region also found significantly lower rates of atherosclerosis risk factors (including hypertension and total cholesterol) and CRP level. Additionally, in patients with Lp(a) levels > 50 mg/dl (125 nmol/l) [33].
Notably, in our analysis, only lower hemoglobin concentration and higher platelet count in multivariate analysis were significantly associated with increased Lp(a) concentrations. Unlike in other studies, in our population, we did not find a correlation of increased Lp(a) concentration with gender or LDL-C [34, 35]. Perhaps the lack of influence of these parameters resulted from the selection of the studied population – very high cardiovascular risk – because most of the research results come from groups with lower cardiovascular risk, and even often from the general and younger population [33, 36].
Study limitations. Like other registries, the Zabrze-Lip(a)R also has some limitations. The first of them is the ability to determine the Lp(a) concentration only quantitatively, and not qualitatively, which in patients with dominant light or heavy Lp(a) isoforms may affect the proper molar Lp(a) concentration. Another limitation is the lack of precise information on the incidence of PAD; hence, it was not included in the results. All other missing data which can now be seen as a limitation will be gradually gathered based on the study design and will be available for the next analysis.
In conclusion, extensive, real-life observational studies are essential for further work that objectively collects information on the risks associated with Lp(a) values and cardiovascular disease. Additionally, developing a deeper understanding of the impact of Lp(a) on prognosis will be valuable in determining how Lp(a) interacts with other established risk factors in improving cardiovascular risk prediction. Finally, it is critical to look for geographical differences that might affect the final CVD risk and Lp(a)-related event predictions. The Zabrze-Lip(a)R will provide real-world data to help answer these crucial questions.