Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / BASIC RESEARCH
Knockdown of microRNA-130b improves doxorubicin sensitivity in bladder urothelial carcinoma by negatively regulating cylindromatosis expression
1
China Medical University, Shenyang, China
Submission date: 2018-03-15
Final revision date: 2018-07-10
Acceptance date: 2018-07-25
Online publication date: 2019-07-17
Publication date: 2021-07-16
Arch Med Sci 2021;17(4):1038-1043
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Chemotherapeutic resistance reduces the sensitivity of bladder urothelial carcinoma (BUC) to chemotherapeutic drugs and contributes a barrier leading to treatment failure. The purpose of this research project is to investigate the regulatory effects of miR-130b on chemotherapeutic drug resistance of BUC and its mechanism.
Material and methods:
The relative expression of miRNA-130b and cylindromatosis (CYLD) was examined using real-time quantitative PCR. The cell proliferation and doxorubicin sensitivity were detected with the enhanced CCK-8 assay. The specific combination of miR-130b and CYLD was verified with the luciferase reporter gene assay. Protein expression was detected by Western blot.
Results:
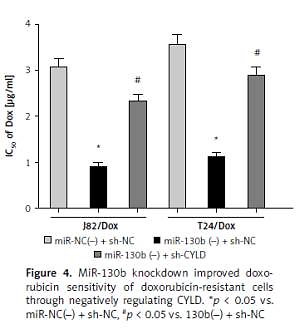
Our study found that miR-130b was up-regulated in doxorubicin-insensitive BUC tissues and cell lines, and its high expression was negatively related to doxorubicin sensitivity in BUC. The miR-130b knockdown reduced the IC50 of doxorubicin and improved doxorubicin sensitivity of J82/Dox and T24/Dox cells. For the regulation mechanism analysis of miR-130b, bioinformatics analysis software was used to predict the potential targets of miR-130b, including the CYLD gene. The following luciferase activities assay, quantitative real time-PCR and western blot identified the CYLD gene as a target of miR-130b. Knockdown of CYLD reversed miR-130b’s regulatory roles in doxorubicin sensitivity in J82/Dox and T24/Dox cells.
Conclusions:
High expression of miR-130b is negatively related to doxorubicin sensitivity in BUC, and knockdown of miR-130b improves doxorubicin sensitivity in BUC by negatively regulating CYLD expression. Our findings will provide guidance for the clinical chemotherapy of BUC.
Chemotherapeutic resistance reduces the sensitivity of bladder urothelial carcinoma (BUC) to chemotherapeutic drugs and contributes a barrier leading to treatment failure. The purpose of this research project is to investigate the regulatory effects of miR-130b on chemotherapeutic drug resistance of BUC and its mechanism.
Material and methods:
The relative expression of miRNA-130b and cylindromatosis (CYLD) was examined using real-time quantitative PCR. The cell proliferation and doxorubicin sensitivity were detected with the enhanced CCK-8 assay. The specific combination of miR-130b and CYLD was verified with the luciferase reporter gene assay. Protein expression was detected by Western blot.
Results:
Our study found that miR-130b was up-regulated in doxorubicin-insensitive BUC tissues and cell lines, and its high expression was negatively related to doxorubicin sensitivity in BUC. The miR-130b knockdown reduced the IC50 of doxorubicin and improved doxorubicin sensitivity of J82/Dox and T24/Dox cells. For the regulation mechanism analysis of miR-130b, bioinformatics analysis software was used to predict the potential targets of miR-130b, including the CYLD gene. The following luciferase activities assay, quantitative real time-PCR and western blot identified the CYLD gene as a target of miR-130b. Knockdown of CYLD reversed miR-130b’s regulatory roles in doxorubicin sensitivity in J82/Dox and T24/Dox cells.
Conclusions:
High expression of miR-130b is negatively related to doxorubicin sensitivity in BUC, and knockdown of miR-130b improves doxorubicin sensitivity in BUC by negatively regulating CYLD expression. Our findings will provide guidance for the clinical chemotherapy of BUC.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.