Introduction
Opioid analgesics are the key element of pain management in cancer patients [1–3]. However, even a few days of opioid treatment may result in severe bowel dysfunction. Opioid-induced constipation (OIC) affects 42.4% of end-stage oncology patients and is one of the most frequent symptoms besides pain and cachexia in these patients [4]. OIC is defined in Rome IV Diagnostic Criteria as new or worsening symptoms of constipation when initiating, changing, or increasing opioid therapy, which must include two or more of the symptoms defining functional constipation. The criteria must be fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis [5, 6]. However, such a long period of observation is not feasible in most palliative care patients because the survival in many cases is much shorter. That is why the Polish Society of Palliative Medicine recommends 7 days of observation as sufficient for the right clinical decision on laxative treatment, which was proven in the validation process to be an effective approach [7].
Constipation is a troublesome adverse effect of opioids because it does not cease, but augments with increased duration of opioid therapy. OIC is difficult to manage, and the effectiveness of regular laxatives is poor [2, 3, 8]. In 85–95% of cases, constipation lowers health-related quality of life, although two-thirds are of mild and moderate intensity [9]. Constipation also generates an additional financial burden for the health system [10].
Poorly responsive OIC is a significant barrier to effective pain management. Every tenth patient requires a change of analgesic, and half of the patients with constipation receive suboptimal analgesia due to gastrointestinal side effects [11].
Opioid-treated patients are more likely to use treatment for constipation compared with those not receiving opioids [12].
It is strongly recommended, both by the European Association for Palliative Care and the European Society for Medical Oncology, that laxatives for the management or prophylaxis of opioid-induced constipation be routinely prescribed [2, 13]. The intensity of preventive laxative use should aim to reach at least three bowel movements per week and to control the intensity of subjective symptoms [7]. An alternative method of prevention may be the use of opioid antagonists such as prolonged-release naloxone combined with oxycodone [14]. Very low-quality evidence suggests that naloxone in combination with oxycodone reduces the risk of constipation without an increase in pain, when compared with oxycodone alone in adults with malignancies and opioid-induced constipation [15].
All opioids induce constipation, although to different degrees. High doses of tramadol are less constipating than small doses of morphine [16, 17]. The frequency of constipation after oxycodone or morphine is similar [18]. Parenteral opioids impair intestinal peristalsis less than oral ones [19]. Transdermal fentanyl and buprenorphine induce bowel dysfunction less often, although it is their most frequent adverse effect [20–22]. However, in large observational cohort studies, the transdermal opioids did not differ from oral opioids in the frequency of constipation [23, 24].
Bowel dysfunction results from both the central and peripheral action of opioids. The central mechanism consists of the activation of neurons in the spinal cord, leading to slower bowel passage and decreased secretions. However, OIC is mainly induced by activation of local μ-opioid receptors in the gut, which are present in the stomach, small intestine, and colon, mainly in the myenteric and submucosal plexus, and on immune cells of the lamina propria [25]. The influence of opioids ultimately sums up to three gastrointestinal effects: a decrease in secretion of fluids, depression of peristaltic contractions and promotion of non-propulsive motility patterns, and the spasm of all sphincters [26]. Dehydration of intestinal contents leads to the occurrence of faecal stones, which are difficult to defecate through the tensed anal sphincter. The gut is overloaded and extended by these hard stool masses [25, 26]. This explains why bulk laxatives are ineffective [13]. On the other hand, osmotic and stimulant laxatives are recommended in OIC. However, their effectiveness is poor because they do not address the opioid receptor-mediated mechanism of bowel dysfunction, so a substantial number of patients do not achieve adequate relief of symptoms [27].
Apart from this, long‐term laxative use can be associated with damage to the muscular function of the bowel; nutritional deficits in terms of loss of water, vitamins, and minerals; and kidney stones or renal failure, in addition to modifying the effects of other medicines [28].
So, an alternative or supplemental method could be adding prokinetics, which might increase the effectiveness of laxatives or opioid antagonists. However, the clinical evidence for this effect is sparse, except for the 5-HT4 receptor agonist prucalopride [29]. Metoclopramide does not act on the gut, and thus should not be considered in constipation.
Itopride is a dopamine D2 antagonist with acetylcholinesterase inhibitory actions. It has a stimulatory action on colonic peristalsis, propelling colonic luminal contents, different from that of cisapride and mosapride, and therefore it may be a useful drug for the treatment of functional constipation [30]. Polish guidelines suggest using prokinetics, such as itopride, for the treatment of constipation [14]. Therefore, some palliative care specialists use it off-label, not only for dyspepsia but also for the management of constipation.
This study aimed to verify whether itopride is effective in the management of opioid-induced constipation in adult palliative care patients in the clinical setting.
The primary outcomes were:
the mean change in the necessity of laxative use in a 0–4 scale assessed after 7 days of treatment; a negative number means a decrease,
the mean number of bowel movements,
the mean intensity of bowel symptoms,
the frequency [%] of constipation, defined as at least one of the following:
The secondary outcomes were:
Material and methods
This open observational study was performed in adult palliative care patients with constipation, who were receiving strong opioids and had not been treated with itopride before the observation. All patients received regular laxatives, and additionally oxycodone/naloxone (OXN group), or itopride (ITP group), or oxycodone/naloxone + itopride (OXN + ITP group). The control group (CTRL) used only laxatives, if necessary.
The inclusion criteria were: legible and complete documentation, age ≥ 18 years, home hospice or inpatient hospice or palliative care outpatient clinic, at least two visits performed in 7–8 day intervals (day 0 and day 7), strong opioids used 7 days before day 0 until day 7, no itopride used up to day 0.
Structured questionnaire
The palliative care practitioners used a questionnaire to report data collected in their medical documentation. The questionnaire included the following items:
Date.
Place (home, ambulatory, hospital palliative care ward, in-patient hospice).
ECOG performance status, assessed by a physician.
Bowel symptoms in the last 7 days, assessed by the patient:
the last defecation [days],
the number of days with bowel movements [days],
the difficulty of defecation in a 0–4 scale, where 0 – no difficulty (“normal defecation”), 1 – mild (“rather normal”), 2 – moderate, 3 – significant/often, 4 – extreme difficulty/always,
too few stools in a 0–4 scale, where 0 – normal stools, 1 – from time to time (mild intensity), 2 – quite often (moderate intensity), 3 – very often, 4 – always,
stools too hard in a 0–4 scale (see above),
the feeling of incomplete bowel movement in a 0–4 scale, where 0 – no symptom, 1 – mild intensity/sometimes, 2 – moderate intensity/quite often, 3 – significant intensity/very often, 4 – extreme intensity/always,
straining or squeezing to try to pass bowel movements, in a 0–4 scale (see above),
the necessity of use of laxatives in a 0–4 scale, where 0 – no laxatives used, 1 – from time to time (occasionally), 2 – often used, 3 – bowel movements only after the use of regular laxatives, 4 – bowel movements only after enema or manual stool evacuation.
The daily doses of opioids, recalculated to the oral morphine equivalent (Table I).
Itopride used 50 mg t.i.d. (yes/no).
Adverse effects.
All the practitioners had participated in a specialisation course on symptom control before data collection. They were aware of the Polish guidelines on prevention and treatment of constipation in palliative care patients. The aim of laxative treatment, according to these guidelines, was to assure bowel movements in the patients taking opioids at least three times a week (preferably every second day) [14].
Table I
Equianalgesic doses of opioids (based on Caraceni et al. 2012 [2])
Source of data
The data were collected in 2018 by 16 palliative care practitioners in 10 (home, inpatient, and ambulatory) palliative care centres for adult patients in Poland. The study received approval of the bioethical committee.
The size of subgroups was one of the outcomes and not to be estimated using statistical methods. We assumed that a minimum of 100 questionnaires collected should be satisfactory to verify the prescribing behaviours regarding the prevention of constipation.
Statistical analysis
Kruskal-Wallis and Mann-Whitney U tests were applied for the statistical analysis of non-parametric data. Frequency analysis was performed using the χ2 and Fisher’s exact tests appropriately. P-values less than 0.05 were considered statistically significant. The data were analysed using Statistica 13 (StatSoft).
Results
The studied population
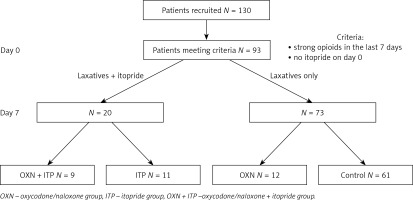
The data of 130 patients were collected. Ninety-two patients met the inclusion criteria (female 54.3%) and were analysed in four groups: OXN + ITP (N = 9), ITP (N = 11), OXN (N = 12), and CTRL (N = 61). The study flow is presented in Figure 1.
The groups did not differ in terms of sex, age, diagnosis, and oral morphine equivalent of strong opioids on day 0 and day 7. The opioids used were as follows: morphine (oral or parenteral), oxycodone, fentanyl, buprenorphine, and tapentadol. The demographic details are presented in Table II.
Table II
The studied population
The symptoms of constipation
The aim of the laxative prevention or treatment was to achieve at least three bowel movements a week and an acceptable intensity of abdominal symptoms, and this aim was achieved in all groups. There were no statistical differences found for the mean values among the groups in terms of particular symptoms of constipation (Table III). Eighty-two (89%) patients had diagnosed constipation, with insignificant differences among groups. However, the anti-constipation strategy appeared successful, on average, in all four groups.
Table III
The intensity of symptoms of constipation
Overall, manual stool evacuation was performed in 4 patients receiving laxatives only, and not in other groups. Enemas were used in 16 patients, and 14 of them were in the control group.
The reduction of laxative use
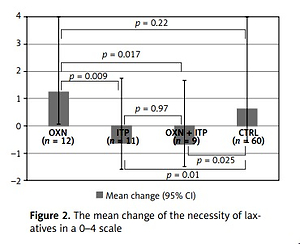
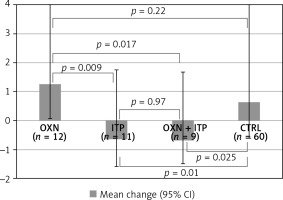
Although there were no statistical differences among groups regarding bowel symptoms or the frequency of bowel movements, the necessity of laxatives decreased in groups in which itopride was used (Kruskal-Wallis ANOVA p = 0.0027). In the ITP group, the necessity to use laxatives decreased by 0.64 (–0.64; 95% CI: –1.6–1.8) and in the OXN + ITP group by 0.67 (–0.67; 95% CI: –1.5–1.7) in the 0–4 scale. These values might seem insignificant, but they were statistically different (Mann-Whitney U test) from oxycodone/naloxone (ITP vs. OXN p = 0.009, OXN + ITP vs. OXN p = 0.017) and the control group (ITP vs. CTRL p = 0.010, OXN + ITP vs. CTRL p = 0.0.25). There was no decrease in laxative use in patients taking oxycodone/naloxone vs. the control group (p = 0.22) (Figure 2).
Adverse effects
This study was a retrospective analysis of the structured history of patients, and none of the 130 patients reported any side effects. This does not mean that there were no adverse effects of treatment, but rather that they were typical and of mild or moderate intensity, so it was not necessary to report them in routine documentation. No patient stopped using itopride between day 0 and day 7. Although the cases in which itopride was used on day 0 were excluded from the analysis, none of the patients stopped itopride use in the next 7 days. Thus, it can be inferred that the medicine was well tolerated.
The history after day 7 was not analysed.
Discussion
This was a non-interventional study, with all limitations that non-randomised clinical trials bear. However, its advantage is a gross effect in the real environment, when using itopride as an element of laxative prevention and treatment. This is probably the first clinical report aiming directly at the use of itopride in OIC.
Physicians did not participate in the analysis and were only asked to provide structured data without specific study goals, to limit possible bias. They delivered the questionnaires regardless of their laxative therapy pattern and use of prokinetics. It also explains the over-representation of a control group, because most patients received regular laxatives, and a minority were treated with oxycodone/naloxone or itopride.
The number of collected cases was sufficient to achieve statistically justified conclusions.
There were no statistical differences between studied groups in terms of age, sex, primary diagnosis, performance status (ECOG), opioids used, or the intensity of symptoms of constipation among the groups.
Following the aforementioned recommendations, every patient receiving opioid analgesics should also receive constipation prophylaxis through the use of laxatives or by using opioids in combination with antagonists (e.g. oxycodone/naloxone) [2, 3]. The Polish guidelines suggest that adding prokinetics may increase the effectiveness of laxative therapy, but there was no direct clinical evidence for that [14]. According to these guidelines, verified in a medium-sized cohort study [7], the prevention of OIC should be provided with an intensiveness that ensures bowel movements at least three times per week and good subjective control of bowel symptoms. This goal was achieved in all studied groups, which did not differ from each other in terms of the intensity of symptoms of constipation. In other words, all four strategies appeared equally effective in the prevention of OIC: (1) itopride added to laxatives, (2) oxycodone/naloxone used with laxatives, (3) oxycodone/naloxone and itopride added to laxatives, or (4) regular laxatives only. It was achieved by intensifying laxative use if necessary.
The main finding of this study is that adding itopride to laxative therapy decreased the necessity to use laxatives. On the other hand, using oxycodone/naloxone did not affect the use of laxatives. What is more, the combined use of oxycodone/naloxone and itopride did not increase the effectiveness of the preventive management of OIC.
The findings confirm the sparse clinical evidence available so far [31].
In conclusion, all strategies of OIC prevention seem equally effective in clinical practice. Itopride, but not naloxone, appeared effective in reducing the use of laxatives in OIC. However, using oxycodone/naloxone or itopride could be associated with decreasing the necessity of interventive and burdensome methods, such as manual stool evacuation or enemas.
The conclusions should be taken with caution because it was not a prospective blinded study. We should treat these results as preliminary. We suggest that an RCT would be valuable to confirm the value of itopride in the strategy of prevention and treatment of OIC.