Introduction
Non-Hodgkin lymphomas (NHL) are heterogeneous group of malignant tumors of hematopoiesis in lymphocytic B or T cell lines. In most cases, these affect lymphatic organs (lymph nodes, spleen and the bone marrow). The indolent – lowly aggressive – types form about 40% of all NHLs [1, 2]. These lymphomas are characterized by slow progression of the disease with many relapses. The inapparent growth often causes the diagnosis to be made in an advanced stage. This group contains diseases such as chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) (essentially the same disease and named CLL/SLL), follicular lymphoma (FL, grade 1, 2 or 3A), lymphoplasmacytic lymphoma (LPL), hairy cell leukemia (HCL), marginal zone lymphoma (MZL) including mucosa-associated lymphoid tissue (MALT) lymphomas, primary cutaneous types including mycosis fungoides, Waldenström’s macroglobulinemia and anaplastic large cell lymphoma (ALCL) [3, 4].
The patient survival rate of indolent lymphomas exceeds 10 years [4, 5]. The composition of the indolent lymphoma tumorous mass becomes, in time, more and more heterogeneous. The mutated cell clones grow faster, have decreased treatment sensitivity, and become a dominant segment of the tumorous mass. This process is named transformation (former terms are blastic transformation and clonal expansion) and has been described in all indolent lymphoma types [6–8]. The most common aggressive arising type is diffuse large B-cell lymphoma (DLBCL). Other aggressive types are follicular lymphoma grade 3B, mantle cell lymphoma (MCL), Burkitt lymphoma, angioimmunoblastic lymphoma, or T-NHL types in general [9]. The transformation of CLL/SLL into aggressive large-cell lymphoma (DLBCL in most cases; Hodgkin lymphoma and T cell lymphomas have been also reported less commonly) is called Richter’s syndrome.
The survival rate in the majority of cases with verified transformation is shorter than 1 year [10]. It is supposed that with the correct selection of the most affected location, a CT-guided core biopsy with a low complication rate and clinical suspicion can establish a final diagnosis without the need of any surgical approach. The aim of our study was to retrospectively evaluate the technical features, efficacy, accuracy, and relevant complications of computed tomography-guided biopsies in the various anatomical localizations when diagnosing indolent lymphoma transformations, relapses, duplicate malignant diseases or benign processes. This information is crucial for the treatment selection, its earliest beginning, and prognosis.
Material and methods
Over the course of 10 years, from December 2007 to December 2017, a group of 72 patients of the Czech Republic population was retrospectively evaluated. The inclusion criteria were the indolent lymphoma type in the patients’ history – CLL/SLL, follicular lymphoma (grade 1, 2 or 3A), lymphoplasmacytic lymphoma, hairy cell leukemia, marginal zone lymphoma, Waldenström’s macroglobulinemia, and anaplastic large cell lymphoma. The biopsies were performed with the clinical or laboratory suspicion of a transformation or relapse and if there was a progressively sized mass on restaging imaging examinations. Other monitored parameters were age, gender, size of the tumor in the longest diameter, localization of the process, biopsy needle gauge, number of biopsy attempts, known different non-lymphoma tumorous diseases in the patients’ history, complications and their solutions, final histological diagnosis, and correlations with results of selected therapy. Also the exclusion of duplicate tumorous process (but in all cases there was indolent lymphoma in patients’ clinical history) was an indication for the interventional procedure.
The biopsies were performed in various anatomical localizations – the face, neck, lung, pleura, mediastinum, chest and abdominal wall, liver, kidney, retroperitoneum, peritoneal cavity, spine, and pelvis. For the statistical data analysis, these localizations were simplified into four subsequent groups – the neck, thorax, abdomen, and pelvis. All of these interventional procedures were performed under CT guidance. The device Siemens Somatom Definition AS Plus (Siemens, Forchheim, Germany) was used. All the patients were indicated by the multidisciplinary council (radiologist, hematologist/hematooncologist or oncologist).
Fully informed consent was obtained in all cases by the biopsy-performing physician with an explanation of the procedure principles, consequences, possible complications, and their solutions. This study was approved by the institutional ethics review committee. The procedures were performed using the Core semi-automatic biopsy system (Bard Magnum Instrument, Bard, Covington, Georgia, USA). In all cases, a one-step approach was used; the coaxial technique was never performed.
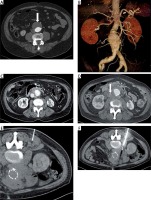
The proper procedure was planned according to a preprocedural restaging imaging examination. The shortest and safest needle distance to reach the lesion was determined. The needle track was planned to also avoid vessels. In one complicated case, a transcaval approach to reach the target process was used. In two cases, the tumorous mass arose in the retroperitoneum in continuity of the inserted aortic stentgraft. The biopsy needle track had to be planned as close to the vessels and also as safe as possible (Figure 1).
Figure 1
Biopsy in the retroperitoneum nearby aortic stentgraft. Aortic aneurysm in transversal plane (A, arrow) on contrast medium enhanced CT and in volume rendering technique reconstruction (B), patient in complete remission of CLL/SLL. Progressive amount of tumorous tissue in the retroperitoneum 24 and 26 months after stent graft insertion (C and D, compressed inferior vena cava is marked with the arrow). The placement of the local anesthesia needle (E) and the biopsy needle (F, patient in prone position). The histological result was diffuse large B-cell lymphoma (DLBCL), i.e. Richter transformation
The entry point was defined by placing a skin mark. The distance and biopsy needle angle were measured on the basis of local anesthesia needle short CT imaging. Skin was disinfected and covered with sterile drapes exposing only the entry site. In accordance with the predetermined route, a 16 G/18 G of 10 cm/13 cm/16 cm length with 22 mm throw needle was inserted in the proper position into the lesion. After obtaining the sample, the needle was carefully removed from the patient’s body. The material was put into a sterile 36–38% formaldehyde solution and the transport system.
All procedures were performed using only local anesthesia (Trimecaine, Zentiva, Prague, Czech Republic); conscious sedation or general anesthesia was never needed. Parameters of blood coagulation, international normalized ratio (INR, lower than 1.5) and activated partial thromboplastin time (aPTT, lower than 1.3) were noted before the biopsy. After the intervention, one series of CT scans in the same ranges as in the preprocedural examination was performed to exclude the possibility of early complications, hemorrhaging being the complication looked for first. In the biopsies affecting pleura (lung interventions) pneumothorax also had to be excluded. The point of insertion into the skin was cleaned with a surgical scrub. The duration of the whole procedure including all preparations never exceeded 30 min. The patients were monitored for the remainder of the day by the standard department of hematology or oncology. The next morning they were discharged from the hospital after clinical, laboratory, and ultrasound examinations. In the thoracic cavity interventions a chest X-ray examination was also completed.
Only 1 patient was not discharged from the hospital the next morning. In this case, pulmonary hemorrhage and hemothorax were revealed immediately after the procedure (Figure 2). The patient’s clinical status was stable, so a conservative approach using analgesia and one blood transfusion was sufficient in this case. The control chest X-ray examinations confirmed non-progressive bleeding. The patient stayed in the hospital for a total of 5 days. In 6 cases of pulmonary conditions, small pneumothoraces were verified, but without clinical correlates. These patients were normally discharged the next morning after the procedure.
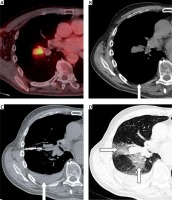
Figure 2
A complication. Viable mass in the right pulmonary hilum on FDG enhanced PET/CT (A), patient with history of follicular lymphoma (grade 2), bronchoscopic examination result was non-diagnostic. The preprocedural CT scan revealed no pleural effusion (B, arrow). The placement of the biopsy needle (C, patient in supine position), hemothorax is present (arrow). Instead of the hemothorax there is also pulmonary hemorrhage (D, arrows), pneumothorax was not revealed. The histological result was melanoma, i.e. duplicate malignancy
According to the tumor location in particular anatomical parts, the patients’ position on the CT table had to be carefully selected. A supine position was suitable for interventions in the neck, anterior part of the mediastinum, abdominal wall and cavity. For the biopsies in the posterior part of the mediastinum, retroperitoneum, spine and dorsal pelvic parts, the patients’ prone side was appropriately positioned. The approach regarding the middle mediastinal, hepatic, renal, and some pelvic processes was special for each particular case. In some cases a percutaneous approach was chosen in the second step in addition to a non-diagnostic attempt of other medical specialties (e.g. bronchoscopy or digestive tract endoscopy).
In conservatively treated patients (transformations, relapses, and benign histological findings) biopsy results were correlated with the findings of the bone marrow evaluation and with the results of the targeted treatment. Conservative treatments with a positive match were considered identical to findings of biopsy results and bone marrow evaluations, or to tumorous tissue amounts that appeared to be significantly decreasing or disappearing completely on follow-up imaging examinations. In surgically treated patients (duplicate malignant affections), histological results were correlated after the biopsy and after the operation approach. It was considered a success when the relationship between the histological results of the biopsy and the surgical resection were identical. For the follow-up, CT (equal CT device) or PET/CT Discovery VCT 64 (General Electric Healthcare, Milwaukee, Wisconsin, USA) was used. In some sporadically indicated situations (e.g. liver conditions), magnetic resonance was performed. Siemens Magnetom Avanto 1.5 Tesla (Siemens, Forchheim, Germany) was used.
If there was a negative histological result and the suspicion of a present tumorous process persisted, a surgical approach for obtaining tissue samples was used. The patients considered for transformed indolent lymphoma and treated without biopsy verifications were not included in the study.
For the study we used retrospective data collection. For the basic quantitative statistical evaluation, median and interval data were used. These parameters were correlated with localizations, complications, number of biopsy attempts, needle gauge, and age using Fisher’s exact test with contingency tables. Qualitative statistical data were descriptively evaluated and quantitative parameters were found. The statistical significance was established at the level p = 0.05. The statistical software NCSS 11 (NCSS, LLC, Kaysville, Utah, USA) was used.
Results
A total of 81 interventional procedures with accessible histological results were included in the study. 81 biopsies in 72 patients were performed. The patients were men in 41 cases (56.9%; 95% CI: 45.5–68.4) and women in 31 cases (43.1%; 95% CI: 31.6–54.5), aged 36 to 86 years (median: 68 years of age). The percutaneous biopsies were performed in various localizations for tumors ranging from 17 to 232 mm (median length: 39 mm). The biopsies were performed in the neck in 4 cases (4.9%; 95% CI: 1.4–12.2), in the thorax in 26 cases (32.1%; 95% CI: 22.2–43.4), in the abdomen in 44 cases (54.3%; 95% CI: 42.9–65.4) and in the pelvis in 7 cases (8.7%; 95% CI: 3.5–17.0). Overall, in only 2 cases were the histological results considered false negative (2.5%; 95% CI: 0.3–8.6). In 79 cases (97.5%, 95% CI: 91.3–99.7) the results were considered true positive or true negative.
The numbers of verified diagnoses and results of cases in particular parts of the body are shown in Table I. Transformation was verified in 29 cases (35.8%; 95% CI: 25.4–47.2), relapses in 30 cases (37%; 95% CI: 26.6–48.5), duplicate malignancy in 15 cases (18.5%; 95% CI: 10.8–28.7) and benign processes in 7 cases (8.7%; 95% CI: 3.5–17.0). The transformations and relapses were proved in all parts of the body. In the thorax, transformation was verified in 8 biopsies, 7 of them in the mediastinum and only 1 in the lungs. For the relapse in 7 biopsies, 6 of them were in the mediastinum and also only 1 in the lungs. On the other hand, thoracic duplicate malignancies and benign diagnoses were proved only in the lung biopsies. Transformation in the abdominal cavity was verified in 15 cases, 11 of them in the retroperitoneum, 3 in peritoneal space, and in 1 case liver impairment was established. In the relapse diagnostics the proportion was similar; out of 19 cases, it occurred in the retroperitoneum 12 times, 6 times in the peritoneal cavity and 1 time the liver was affected.
Table I
The number of result types in particular parts of the body
| Part | Transformation | Relapse | Duplicate malignancy | Benign diagnosis |
|---|---|---|---|---|
| Neck | 2 | 2 | 0 | 0 |
| Thorax | 8 | 7 | 8 | 3 |
| Abdomen | 15 | 19 | 6 | 4 |
| Pelvis | 4 | 2 | 1 | 0 |
| N | 29 | 30 | 15 | 7 |
The 16 G needle was utilized in 69 biopsies (85.2%; 95% CI: 77.4–92.9) and the 18 G needle in 12 interventions (14.8%; 95% CI: 7.9–24.4). The 16 G needle was used in all cases in the neck and pelvis region. The 16 G needle was also sufficient for 42 out of 44 cases in the abdominal region, and an 18 G needle was used in only two risky affected areas located near great vessels. In the thorax, a 16 G needle was used in 11 biopsies in the mediastinum and in 5 cases of lung diseases, and an 18 G needle was used in one biopsy in the mediastinum and in 9 cases of affected lung areas. A schematic overview is presented in Table II. Fisher’s exact test proved a statistically non-significant relationship between the needle gauge and the accuracy of the histological results (p = 0.09481).
Table II
The number of complications and needle gauge in particular parts of the body
| Part | Needle gauge | Complications | ||
|---|---|---|---|---|
| 16 G | 18 G | 16 G | 18 G | |
| Neck | 4 | 0 | 0 | 0 |
| Thorax | 16 | 10 | 3 | 4 |
| Abdomen | 42 | 2 | 0 | 1 |
| Pelvis | 7 | 0 | 0 | 0 |
| N | 69 | 12 | 3 | 5 |
The number of biopsy attempts was variable. One sampling in 11 cases (13.6%; 95% CI: 7.0–23.0), two samplings in 49 interventions (60.5%; 95% CI: 49.0–71.2), three samplings in 16 biopsies (19.7%; 95% CI: 11.7–30.1) and four samplings in 5 cases (6.2%; 95% CI: 2.0–13.8) were performed. One attempt was determined for lung conditions of a small amount and risky interventions near great vessels across all the body. For the majority of interventions, two samples were sufficient. Three or four biopsy attempts were optimal for peripherally located areas and large masses in the thoracic and abdominal cavities with an emphasis on targeting various tumor parts. Fisher’s exact test proved a statistically significant relationship between the number of biopsy attempts and the histological accuracy result (p = 0.02807).
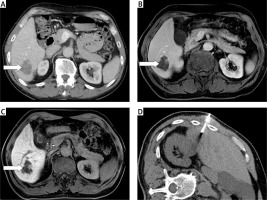
False negative results were confirmed in 2 patients. In both cases, the biopsy revealed CLL/SLL relapse, but clinical suspicion of transformation was still high. The patients were indicated for abdominal diagnostic surgical biopsies with wide tumorous tissue excision; the final histological results confirmed diffuse large B-cell lymphoma (DLBCL), i.e. Richter transformation. The percutaneous biopsy technique was usual, but the samples were obtained from the non-transformed parts of the process (Figure 3).
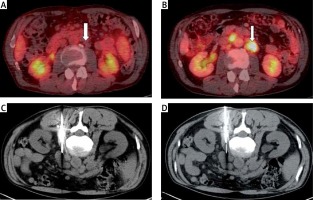
Figure 3
A case of a false negative result. The patient is in long-term remission of CLL/SLL, no significant lymphatic nodes are revealed on the PET/CT examination in transversal plane (A, arrow). The viable mass in the retroperitoneum appeared more than 7 years after initial diagnosis on PET/CT examination in transversal plane (B, arrow). The two attempts of the biopsy (C and D); the histological result was CLL/SLL relapse. The clinical and laboratory suspicion of transformation persisted, the patient was operated on and the final result confirmed diffuse large B-cell lymphoma (DLBCL), i.e. Richter transformation
All true positive results were considered as the final diagnoses. Within the follow-up examinations, the therapeutic success was observed. The verification of duplicate malignant tumorous diseases was sufficient for the oncologists. These verified tumors were small in amount at the time of diagnosis and were resected in the surgical department of the hospital. The histological results of the resected tumors and the biopsies were compared. If there was malignant disease in patients’ clinical history, the histological results were also compared.
Duplicate malignancies were diagnosed in 8 cases in the thorax, in 6 cases in the abdomen, and in 1 case in the pelvis biopsies. Final histological results in the thorax were primarily non-small cell lung carcinomas (NSCLC) – adenocarcinoma in 3 cases and squamous cell carcinoma in 3 cases (Figure 4). In addition, there was 1 case of melanoma and metastatic colorectal carcinoma. Metastatic colorectal carcinoma was diagnosed in the abdomen in 2 cases, and in 1 case cholangiocarcinoma, gastrointestinal stromal tumor (GIST), renal cell carcinoma (papillary type), and renal cell carcinoma (clear cell type) with sarcomatoid features. One case of metastatic colorectal carcinoma changing to lymphatic nodes was histologically verified in the pelvis.
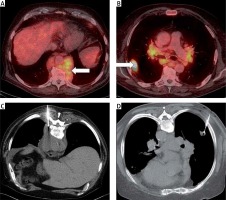
Figure 4
Duplicate malignant process. Viable mass in the posterior mediastinal part (A, arrow) and right middle pulmonary lobe (B, arrow) on FDG enhanced PET/CT, patient with history of CLL/SLL. The biopsy was performed in one step from the mediastinum (C, patient in prone position) and from the right lung (D, patient also in prone position). The histological result from the mediastinum was CLL/SLL relapse and from the lung squamous cell pulmonary carcinoma
In the true negative results, a long-term follow-up was performed. The shortest observation interval was 24 months after the biopsy. In the thoracic cavity, pulmonary interventions were performed, finding histological results of benign pneumonia in 3 cases, which had disappeared at follow-up CT examinations. Benign results in the abdomen included extraadrenal myelolipoma, myelodysplastic syndrome associated with immunosuppressive therapy, liver focal fatty lesions, and liver echinococcal cyst in 1 case (Figure 5). The last mentioned diagnosis was confirmed by surgical resection.
Figure 5
A case of a true negative result. Hypovascularized focus in the right liver lobe on contrast medium enhanced CT (A, arrow) in the patient with the history of marginal zone lymphoma. Magnetic resonance with liver-specific contrast medium administration was performed. The lesion did not contain hepatocytes (portal venous phase – B, and delayed phase – C, arrows). The placement of the biopsy needle (D, patient in left side position). The histological result was echinococcal cyst
The complications of the biopsies were observed and correlated with the needle gauge, number of attempts, and anatomical part of the body (Table II). The statistical data analysis revealed no significant relationship between complication incidence and the number of biopsies and needle gauges. However, the anatomical mass located in the thorax (and especially lungs) has a statistically significantly higher incidence of complications. It was revealed that in a total of 8 complications, 7 of them involving thoracic cavity interventions, only 1 hemorrhage was detected in the retroperitoneum. In the thorax, a total of 26 biopsies were performed; the complication rate was revealed to be 26.9% (95% CI: 11.6–47.8). Fisher’s exact test proved a statistically significant relationship between the complication incidence and anatomical localization (p = 0.0104).
Discussion
We report our results regarding development of the indolent lymphoma types and the role of CT-guided biopsy using a percutaneous approach across almost the whole body. The overall diagnostic accuracy is high; the histological results were true positive in 97.5%. The numbers of verified transformations and relapses were comparable (35.8% and 37%), while duplicate malignancies and benign processes were diagnosed less frequently (18.5% and 8.7%). In 85.2% of interventions we used a thicker biopsy needle of 16 G. In the majority of cases (86.4%) at least two biopsy attempts were made. The thoracic cavity biopsies had a significantly higher complication rate than other anatomical localizations.
In the literature, there are articles about lymphoma diagnostics or subtyping using various imaging guidance, approaches and needle gauges [11–13]. The first published studies yielded 84–88% overall accuracy with subtyping rates of 76–85% [14, 15]. Recently published studies using improved methods declared overall and also subtyping accuracies exceeding 95% [11, 16]. These results are equal to those of surgical excision biopsies. Also our results included in this paper correspond with these published data. However, this information is related to establishing the initial diagnosis, not to lymphoma transformation or relapse. Using percutaneous biopsy instead of surgical excision is a novel modern approach. Long-term comparable data are not available in the literature.
The European Society for Medical Oncology (ESMO) clinical practice guidelines recommend that, when feasible, surgical excision biopsies are the ideal methods for diagnosis, subtyping, grading, and other histological features of malignant lymphomas [17]. The surgical approach is considered a gold standard in peripherally located palpable masses. Excision of the affected lymph node is an optimal situation for the pathologist and extensive histological analysis because of sufficient amounts of tissue to examine. To obtain relevant tissue samples from deeply located tumors (mediastinum, retroperitoneum, mesentery, abdominal cavity and pelvis) a surgical approach was necessary until recent years [18]. This approach resulted in postoperative pain, prolonged recovery time, and the need of general anesthesia [19]. Non-diagnostic results were also verified [20].
To eliminate these disadvantages, minimally invasive techniques using imaging guidance were developed. To do this, the high diagnostic accuracy had to be preserved. The cytology and aspiration biopsy techniques (fine needle aspiration biopsy – FNAB) are not suitable for lymphoma diagnostics or subtyping [21]. The core needle biopsy (CNB), as the interventional method able to repeatedly collect sufficient tumorous tissue amounts, has gradually been accepted as an alternative for lymphoma diagnosis of peripherally and also deeply located conditions [11]. The diagnosis can be established and includes subtyping. The technique is well tolerated, rapid, inexpensive, and this is all with a low complication rate, using only local anesthesia.
Histological transformation is the worse possibility in the indolent lymphoma course. The onset of transformation is heralded by sudden general clinical deterioration, marked increase of lymphadenopathy at one or more locations (often in the abdominal cavity), splenomegaly, and the expression or worsening of “B” symptoms, i.e. fever, night sweats, and weight loss. Also, some laboratory findings are related to transformation – elevation of the serum level of lactate dehydrogenase (LDH), anemia, and thrombocytopenia [5, 17]. Some risk factors have also been defined for transformation development: they can only slightly differ among particular histological types. Eastern Cooperative Oncology Group (ECOG) performance status > 1, involvement of more than one extranodal structure, histological grade 3 or 4, lack of complete remission following initial treatment, and decreased serum level of albumin or increased serum level of β2-microglobulin are some of the reported risk factors [5, 22]. Several studies include cases with transformation diagnosed following clinical and laboratory criteria [9]. It is not believed that the presence of at least one of the mentioned criteria is sufficient to reliably identify transformed patients, because the same parameters are generally associated with advanced indolent lymphomas and correspond with poor outcomes, also without the presence of transformation [23].
The imaging methods have an irreplaceable position in the restaging of indolent lymphomas. The neck, thorax, abdomen, and pelvis computed tomography (CT) using intravenous contrast medium administration and especially 18F-fluoro-2-deoxyglucose (18F-FDG) labeled positron emission tomography/computed tomography (PET/CT) can determine the character, size, or amount of lymphadenopathy and spleen, correlate it with previous examinations, find appropriate extranodal conditions, and define the status of the disease [24]. The localization of increased amount of lymphadenopathy moreover with a high level standardized uptake value of 18F-FDG is the preferred site for the biopsy. This information plays a crucial role for high diagnostic accuracy.
In the literature, there is a positive correlation between using a larger needle gauge and improved diagnostic yield. In general, a larger core size allows for more tumorous tissues to be collected and examined. Using a larger needle gauge (14 or 16 G) is completely suitable for peripherally located palpable masses, conditions of the anterior and posterior mediastinum, and the abdominal cavity including retroperitoneum and pelvis. Thin needle gauges (18 G) are satisfactory for interventions affecting the pleura due to the sufficient diagnostic accuracy combined with an acceptable complication rate [25].
The lymphoma patients’ history and surveillance need close multidisciplinary cooperation. All specialties (hemato-oncology, radiology, and pathology) should be experienced in specific lymphoma issues. The key role of the clinician is to recognize the relapse or transformation possibility. The role of the radiologist is to perform the restaging imaging examination as soon and precisely as possible, determine the current status, mark the most suspected localization, and perform safe and sufficient percutaneous biopsies. The CT or PET/CT is preferred prior to other imaging methods due to depicting almost the whole body and finding the most progressive tumorous site. The most suitable location for a biopsy is the mass with significantly increased metabolic aktivity detected using PET/CT. Making the correct decision for the best biopsy location is crucial for high diagnostic accuracy and true positive results. In almost all indolent lymphoma cases different locations are affected. The biopsy or surgical excision in a more accessible, but not the most progressive location, although technically excellent, then leads to false negative histological results.
The limits of our study are the retrospective data analysis, which depends on the hospital information system, smaller number of patients (but comparable with published papers), absence of a control group and the individual indications depending on the actual specific patients’ status evaluation. The upcoming treatment depends on the histology – the therapeutic approach to lymphoma relapse or transformation, duplicate malignancy or benign disease will be completely different.
In conclusion, core needle biopsy using CT guidance and the percutaneous approach has high diagnostic accuracy not only in establishing the initial lymphoma diagnosis, but also in lymphoma development – transformation to aggressive types, relapses, duplicate malignant diseases or benign (e.g. inflammatory) processes. Core needle biopsies, using at least two samplings of the 16 G or 18 G needle, the most suitable location depending especially on restaging imaging examinations, have a low complication rate (except thoracic interventions), and should be utilized in all parts of the lymphoma diagnostics, and across almost the whole body.


