Introduction
Rheumatoid arthritis (RA) is a hereditary, auto-inflammatory disease that causes chronic synovitis, leading to cartilage and bone destruction, and approximately 1–2% of the population are affected [1]. The growth of synovial tissue (pannus) towards the subchondral bone above and below the joint cartilage surface has been associated with the destruction of the related joints [2, 3]. Synovial cells are responsible for joint damage and the conditions that form through the expression of degrading enzymes, including several inflammatory cytokines and growth factors, prostaglandins [4], collagenase [5], stromelysin [6], and cathepsin L, B, and D [7].
Pentraxins are inflammatory markers of multi-functional protein groups containing long pentraxins such as pentraxin 3 (PTX3) and short pentraxins such as serum amyloid P and C-reactive protein (CRP) [8]. CRP is produced in the liver after interleukin-6 (IL-6) stimulation, whereas PTX3 is produced locally in the inflammation region after stimulation with pro-inflammatory cytokines such as tumour necrosis factor (TNF) and interleukin-1β (IL-1β) [9, 10]. Pentraxin 3 is produced in many cells such as dendritic cells, monomorphonuclear and polymorphonuclear phagocytes, fibroblasts, adipocytes, endothelial cells, synoviocytes, and alveolar epithelial cells and plays a role in various biological processes such as female fertility, extracellular matrix deposition, inflammation, and immunity [11–13]. In physiological conditions, apart from in the pre-ovulatory period and during pregnancy, the plasma PTX3 level is very low (< 2 ng/ml in humans, < 25 ng/ml in mice). While the PTX3 level is very low in healthy human organs, with the exception of the ovaries, under inflammatory conditions, PTX3 expression increases [10].
Pentraxin 3 in the serum has been related to disease activity in the vascular compartment and cardiovascular diseases (CVD) [14] and in vasculitis [15–18]. Pentraxin 3 levels have been examined in various rheumatic and autoimmune diseases such as cutaneous psoriasis, systemic sclerosis, many inflammatory vascular diseases, multiple sclerosis, IgA glomerulonephritis, RA, ankylosing spondylitis (AS), and tuberculosis [19–25].
The aim of the current study was to identify whether serum PTX3 level could be a potential marker of increased inflammation in RA patients. We compared serum PTX3 levels between RA patients and a healthy control group; the relationship between PTX3 level and disease activity was also examined.
Material and methods
The study included 41 patients diagnosed with RA according to the American College of Rheumatology (ACR) revised 1987 diagnostic criteria [26]. They were recruited from the Physical Therapy and Rehabilitation Polyclinic of Bozok University, and all patients were receiving disease-modifying anti-rheumatic drugs (DMARDs). The control group comprised age- and gender-matched healthy individuals.
All patients were evaluated in respect of age, body mass index (BMI), complete blood count (CBC), leukocytes, platelets, mean platelet volume (MPV), erythrocyte sedimentation rate (ESR), CRP values, rheumatoid factor, anti-cyclic citrullinated peptides (CCP), iron, ferritin, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, ferritin, vitamin D, and vitamin B12. The disease activity scores of the patients were calculated with DAS 28. Patients with RA were divided into three groups according to the DAS28: low: 2.6 – < 3.2; mild: 3.2 – ≤ 5.1; and high: > 5.1.
Exclusion criteria were: acute infection, the presence of other rheumatic diseases, severe renal, liver, and cardiac diseases, atherosclerotic CV disease (i.e. coronary artery disease, history of acute coronary syndrome, stroke/transient ischaemic attack, peripheral artery disease, and symptomatic carotid artery stenosis), chronic obstructive pulmonary disease, and malignancy.
The study was approved by Bozok University Medical Faculty Ethics Review Board (no. 2016-02/06). Written, informed consent was obtained from each participant. This research complied with the principles of the Declaration of Helsinki.
Biochemical measurements
For the biochemical measurements, blood samples were drawn after a fasting period of 12 h. Plasma was immediately separated by centrifugation. Routine laboratory tests such as CBC test, CRP, ESR, serum levels of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, ferritin, vitamin D, and vitamin B12 were determined. MPV was measured in a blood sample collected in citrate, in order to avoid the platelet swelling induced by EDTA. A Sysmex XN-1000 haematology analyser was used for whole blood counts. The expected values for MPV in our laboratory ranged from 5.0 to 15 fl. Platelet mass was calculated as the MPV – platelet count.
Pentraxin 3 protein determination by ELISA
Blood samples were collected in Vacutainer tubes without anticoagulant supplements. All blood samples were centrifuged (3000 rpm, 10 min), and the supernatant was immediately removed and stored at –80°C until assayed by an investigator blinded to the groups. Pentraxin was assessed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (HK347 Human Pentraxin 3, Hycult Biotech; Netherlands). Serum pentraxin 3 concentrations are expressed in ng/ml.
Statistical analysis
Statistical analyses of the study data were performed with SPSS 17.0 software. Categorical measurements were stated as number (n) and percentage (%) and continuous variables as the mean ± standard deviation (SD), or where necessary as median and minimum and maximum values. In the comparison of the continuous measurements according to groups, distribution of the data was evaluated, and variables conforming to the parametric test were applied with the Student’s t-test, and for those not conforming, the Mann Whitney U-test was used. In the comparison of categorical variables, the χ2 test or Fisher’s exact test were used. Pearson’s correlation test was used to evaluate the relationship between variables. Independent risk factors were evaluated using logistic regression analysis, but no significant risk factor was determined. In all the tests, a value of p < 0.05 was accepted as statistically significant.
Results
Forty-one patients were studied with mean age 46.0 ±13.4 years, 30 (73.2%) were female and 11 (26.81%) male, as well as 42 healthy volunteers, as controls, with mean age 42.2 ±13.5 years, 24 (57.1%) female and 18 (42.9%) male. There was no significant difference between RA patients and controls as regards age and sex (p = 0.2 and 0.16, respectively). Disease activity (DAS28) was low for 25, mild for 11, and high for 5 patients. Fifteen patients had anti-CCP positivity, whereas 26 patients had negative anti-CCP.
The laboratory test results of the patient and control groups are shown in Table I. A statistically significant difference was determined between the groups in respect of the PTX3, PLT, CRP, and MPV results (p = 0.042, p = 0.007, p = 0.017, p < 0.001, respectively) (Table I). Mean PTX3 in RA patients with low, mild, and severe disease activity and in anti-CCP-positive and -negative patients are shown in Tables II and III, respectively.
Table I
Laboratory test results of patient and control groups
| Parameters | Patients (n = 41) | Controls (n = 42) | P-value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (min.–max.) | Mean ± SD | Median (min.–max.) | ||
| Age | 46.0 ±13.4 | 47 (25–86) | 42.2 ±13.5 | 38 (17–82) | 0.201 |
| BMI | 27.5 ±5.9 | 26.3 (18.5–45) | 25.9 ±4.8 | 25 (18.4–38.0) | 0.178 |
| PTX3 [ng/ml] | 0.019 ±0.05 | 0 (0–0.29) | 0.007 ±0.03 | 0 (0–0.23) | 0.042* |
| Leukocyte [103/mm3] | 7.6 ±1.9 | 7.48 (3.6–12.0) | 7.8 ±2.5 | 7.4 (3.4–15.7) | 0.806 |
| MPV [fl] | 6.8 ±1.2 | 7.95 (6.0–9.7) | 7.9 ±0.9 | 7.98 (6.4–9.9) | 0.001* |
| HB [g/dl] | 13.3 ±1.4 | 13.3 (10.9–16.1) | 13.7 ±1.8 | 13.7 (9.4–17.2) | 0.343 |
| PLT [103/mm3] | 303.7 ±80.5 | 304 (132–451) | 264.5 ±61.9 | 253 (147–464) | 0.007* |
| ESR [mm/h] | 18.6 ±11.2 | 16 (1–44) | 14.0 ±8.7 | 13 (1–33) | 0.063 |
| CRP [mg/l] | 10.6 ±11.1 | 6.3 (0.1–48.7) | 6.8 ±11.4 | 2.9 (0.1–50.6) | 0.017* |
| B12 [pg/ml] | 288.0 ±94.2 | 280 (126–529) | 315.5 ±153.2 | 277 (130–696) | 0.809 |
| DVit [nmol/l] | 22.0 ±13.0 | 19.3 (5–58) | 19.9 ±10.2 | 17.6 (7–49) | 0.591 |
| TC [mmol/l] | 238.8 ±83.0 | 219 (100–436) | 265.1 ±72.5 | 257 (145–436) | 0.110 |
| TG [mmol/l] | 197.2 ±59.6 | 198 (76–278) | 203.1 ±64.0 | 209 (73–321) | 0.619 |
| LDL-C [mmol/l] | 198.0 ±251.5 | 138.6 (64–1644) | 169.6 ±159.5 | 135.3 (47–1098) | 0.636 |
| HDL-C [mmol/l] | 54.4 ±62.9 | 45 (12–438) | 43.6 ±11.2 | 42.5 (26–72) | 0.480 |
| Fe [mg/dl] | 53.1 ±28.4 | 50 (5–150) | 59.5 ±31.1 | 57 (21–198) | 0.301 |
| Ferritin [ng/ml] | 40.3 ±22.9 | 35 (5–96.7) | 56.6 ±42.9 | 50.3 (7.6–204) | 0.087 |
Table II
Pentraxin 3 values according to disease activity level
| Disease activity | Number of patients | Mean | Standard deviation | Maximum |
|---|---|---|---|---|
| Low | 25 | 0.022 | 0.065 | 0.29 |
| Mild | 11 | 0.008 | 0.025 | 0.08 |
| High | 5 | 0.036 | 0.049 | 0.09 |
Table III
PTX3 values in anti-CCP-positive and -negative patients
| Anti-CCP | Number of patients | Mean | Standard deviation | Maximum |
|---|---|---|---|---|
| Positive | 15 | 0.0275 | 0.0663 | 0.2940 |
| Negative | 26 | 0.0066 | 0.0218 | 0.0840 |
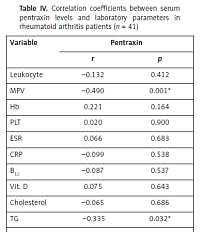
A correlation was determined between PTX3 and MPV (r = –0.490, p = 0.001) and TG (r = 0.335, p = 0.032). There was no statistically significant correlation between PTX3 and ESR (r = 0.066, p = 0.683) and PTX3 and CRP (r = 0.099, p = 0.538) in the RA group. Correlation analysis between PTX3 and all studied parameters in RA patients are shown in Table IV.
Table IV
Correlation coefficients between serum pentraxin levels and laboratory parameters in rheumatoid arthritis patients (n = 41)
| Variable | Pentraxin | |
|---|---|---|
| r | p | |
| Leukocyte | –0.132 | 0.412 |
| MPV | –0.490 | 0.001* |
| Hb | 0.221 | 0.164 |
| PLT | 0.020 | 0.900 |
| ESR | 0.066 | 0.683 |
| CRP | –0.099 | 0.538 |
| B12 | –0.087 | 0.537 |
| Vit. D | 0.075 | 0.643 |
| Cholesterol | –0.065 | 0.686 |
| TG | –0.335 | 0.032* |
| LDL | –0.026 | 0.872 |
| HDL | 0.143 | 0.373 |
| Fe | –0.031 | 0.848 |
| Ferritin | 0.049 | 0.760 |
| RF | 0.080 | 0.620 |
There was no statistically significant difference in PTX3 levels between anti-CCP-positive and -negative patients (p = 0.368). No statistically significant difference was determined in respect of PTX3 levels in RA patients according to disease activity (p = 0.346).
Discussion
The aim of this study was to determine whether serum PTX3 levels could be a potential marker of chronic inflammation in RA patients. Therefore, the serum PTX3 levels of RA patients were compared with those of healthy individuals, and the relationship between PTX3 levels and disease activity was evaluated. The results showed that although there was a statistically significant difference in the PTX3 levels of RA patients compared to those of the healthy control group, no significant relationship was determined with disease activity. No difference was determined between seronegative and seropositive RA patients in respect of PTX3 levels.
Although not specific, CRP and ESR are widely used as clinical markers of inflammation and of acute phase response for identification of rheumatological diseases and for follow-up [27, 28]. PTX3, similarly to CRP, is a pattern recognition molecule of the immune system and has many important functions, including anti-microbial effects, participation in the clearance of apoptotic cells, and regulation of inflammation [29]. Previous studies have shown that, compared with control groups, PTX3 levels are high in various rheumatic diseases, such as RA [30], PsA [31], AS [32], giant cell arteritis [15], SLE [16], and small vessel vasculitis [17].
It has been determined in previous studies that PTX3 levels are high in RA patients with cardiovascular disease [33]. In patients with small vessel vasculitis, PTX3 levels have been reported to be higher, although no correlation has been determined between PTX3 and CRP [17].
In a study by Kahlow et al. [34], the PTX3 levels and carotid intima media thickness values were examined in RA patients, and PTX3 levels were not shown to reflect inflammation, and no relationship was determined with DAS28. Although not specific, CRP is thought to be the most useful indicator of inflammation in RA, and no association has been found between PTX3 and other inflammatory markers, such as ESR and CRP. Similarly to other reports in the literature, the results of the current study determined no correlation between PTX3 levels and disease activity, SED, or CRP in RA patients.
In a study by Tekeoglu et al. [30], the relationship was investigated between disease severity, clinical characteristics, ESR and CRP, and potential biochemical markers of inflammation (PTX-3, IL-6, fetuin-A, insulin, and insulin resistance). It was reported that elevated levels of PTX3, fetuin-A, CRP, and ESR could play a role in the pathogenesis of RA. No relationship was found between the clinical severity of RA and PTX3, CRP, and ESR levels. Similarly, in the current study no relationship was determined between disease activity and PTX3 levels.
In RA patients with high levels of RF or anti-CCP, an increased risk of joint collapse and a more severe disease course compared to seronegative patients has been reported [35, 36]. In the current study, no difference was determined between seropositive and seronegative RA patients in respect of PTX3 levels.
In a recent meta-analysis, which evaluated a total of 20 studies, comprising seven on SLE, four on AS, five on RA, three on SSc, and one on MS, the results showed that serum/plasma PTX3 levels of patients were significantly high compared to those of normal control groups [37]. In the current study, the PTX3 levels of the RA patients were found to be significantly high compared to those of the control group, which was consistent with the findings in the literature.
The MPV levels of the RA group in the current study were determined to be low, compared to those of the control group, and there was a negative correlation with PTX3. To the best of our knowledge, there has been no previous study showing a correlation between MPV and PTX3. Previous studies have demonstrated that thrombopoietin and innumerable inflammatory cytokines (e.g. IL-1, IL-6, and TNF-α) regulate thrombopoiesis, and MPV reflects both pro-inflammatory and pro-thrombotic events. The relationship of systemic inflammation intensity with large and small thrombocytes in circulation seems to be a differentiating factor [38]. In similar studies, evidence has been shown that low MPV plays an important role as an indicator of the efficacy of anti-inflammatory treatment and disease activity in several chronic inflammatory diseases such as RA, AS, familial Mediterranean fever, acute rheumatic fever, and osteoarthritis [38–41]. In the current study, a negative correlation was determined between PTX3 and MPV, which is thought to be associated with inflammation.
There were some limitations to this study. The conclusions were constrained by the low number of study participants, and no comparison was made with any other markers of inflammation. All the patients were receiving DMARD treatment, and no comparison was made of the PTX3 levels before starting the medical treatment.
In conclusion, no relationship was determined between PTX3 and disease activity in RA patients, nor with traditional biochemical measurements of disease activity. However, the PTX3 levels of the RA patients were found to be high in comparison with the control group. The primary sources and triggers of increased PTX3 levels in RA are not very clear. PTX3 may be found in various cells that are part of the local inflammatory process; for example, it may originate from neutrophils in circulation, PLT, joints, and from the cardiovascular system. Because, from these results, the role of PTX3 in the pathogenesis of RA cannot be ignored, there is a need for further studies to determine the potential triggering role of the production of PTX3.