Introduction
Primary Sjögren’s syndrome (pSS) is a chronic, systemic autoimmune disorder manifested as the so-called sicca syndrome, e.g. dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia) [1]. While its pathogenesis is not completely understood, lymphocytes play a critical role in the development of pSS. Generally speaking, pSS has a favorable prognosis, whereas life-threatening situations do occur when pulmonary, renal and nervous systems are inflicted [2]. Primary Sjögren’s syndrome is also associated with an increased risk of malignancy, especially non-Hodgkin’s lymphoma (NHL) [3]. Serological examination often demonstrates the presence of autoantibodies such as anti-Sjögren-syndrome-related antigen A (SSA; Ro) antibodies and anti-Sjögren-syndrome-related antigen B (SSB; La) antibodies. The frequency of anti-Ro/SSA and/or anti-La/SSB antibodies is 60–80% and 30–40%, respectively [4]. The pooled incidence is 0.06% [5] with a female predominance (between 20 : 1 and 9 : 1) [6].
To date, no drug has shown consistent and optimal efficacy for the treatment of pSS; therefore, there are many resistant and refractory patients. In addition to tropical palliative managements, conventional disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate (MTX) and anti-malarial drugs such as hydroxychloroquine (HCQ) are often used in patients with musculoskeletal inflammation. Severe cases with extraglandular involvement may be treated with glucocorticoids [7]. Of note, no randomized controlled trials (RCTs) have been performed to support the current treatments. As the applications of biological agents in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) have shown impressive outcomes, it is reasonable to postulate that these agents, especially those targeting lymphocytes, may improve the efficacy for resistant and refractory patients. In this review, we summarize the currently available biological agents for pSS and explore the possible use of some novel treatments.
Material and methods
We performed a comprehensive literature search for relevant articles using PubMed and the following MeSH terms: “Sjögren’s syndrome”, “biologic therapies”, “T cell targeted therapy”, “B cell targeted therapy”, “abatacept”, “baminercept”, “belimumab”, “rituximab” and “epratuzumab”. We only selected English publications and included randomized controlled trials, observational studies, clinical trials and case reports. Reference lists of relevant articles were reviewed as well. The search was performed with no date limits and last updated on May 2019. We also searched for the relevant ongoing clinical trials in ClinicalTrials.gov.
Pathogenesis
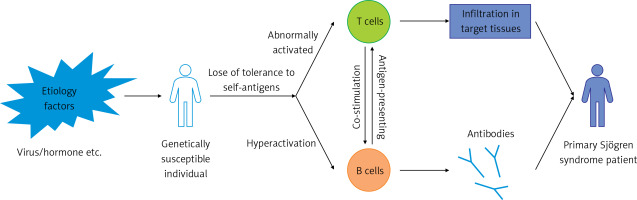
The pathogenesis of pSS is not completely understood. The etiology is multifaceted with the involvement of genetic, epigenetic and environmental factors [8]. While both B cells and T cells play a pivotal role in its initiation and progression, their roles differ at different stages of pSS. Some cytokines including B-cell activating factor (BAFF), tumor necrosis factor α (TNF-α), interleukin-1 (IL-1) and IL-6 are also involved in these processes [9]. Collectively, these elements cause the formation of pSS (Figure 1), and novel therapeutic targets may be generated based on in-depth understanding of these mechanisms.
Figure 1
The initiation and pathogenesis of primary Sjögren’s syndrome (pSS). Auto-reactive lymphocytes, including T cells and B cells, arise when environmental factors such as viruses and hormone imbalance act on genetically susceptible individuals. T cells and B cells play different roles in the commencement and maintenance of pSS. The interaction between T cells and B cells is also crucial in the process of autoimmunity
T cells in the pathogenesis of primary Sjögren’s syndrome
T cells are the dominating components of lymphocyte infiltration in exocrine glands of pSS patients and a major player in the initiation or early stage of disease development [10]. Since most T cells in glandular infiltrates of pSS patients are CD4+ cells [11], we focus on this set of T helper (Th) cells. After aberrant activation by multiple factors, CD4+ Th cells differentiate into a variety of subsets and participate in pSS pathogenesis and development by regulating different autoimmune processes.
Previous studies have demonstrated an imbalanced ratio of Th1 and Th2 cells with a Th1 predominance in pSS. Th1 cells are primary secretors of interferon γ (IFN-γ), a type II interferon able to incite pSS formation [9]. Th17 cells have a profound role in the structure and function of epithelial cells, the formation of ectopic germinal centers (EGCs) and pathogenesis of pSS by producing the proinflammation cytokine IL-17. Several lines of evidence suggest that in addition to Th17 cells, several other subsets of T cells, such as the CD4– CD8– double negative T cells, can also produce IL-17. Collectively, these cells are referred to as “IL-17-producing T cells” and targeting these cells may help the development of new treatments for pSS [12]. However, the role of regulatory T (Treg) cells in pSS still remains elusive. A recent study revealed the existence of a subset of salivary-gland-protective Treg cells in male non-obese diabetic (NOD) mice, and these cells protected salivary glands from damage by autoimmune responses. Interestingly, this subset of Treg cells was not detected in female NOD mice [13]. These observations may partially explain the female predisposition to pSS and the immunosuppressive effect of Treg cells in autoimmunity. Another subset of T cells, i.e. T follicular helper (Tfh) cells, also referred to as B cell helper T cells, coordinates T and B cell crosstalk during pSS development. Tfh cells promote T cell-dependent B cell responses by secreting IL-21, a crucial cytokine causing B cell activation and differentiation into plasma cells [12].
B cells in primary Sjögren’s syndrome
The role of B cells becomes predominant in the advanced stage of pSS [14]. B cells participate in autoimmune responses via several mechanisms: producing various cytokines, presenting antigens and generating autoantibodies [8].
B cell hyperactivity is a key mechanism underlying pSS pathogenesis as evidenced by characteristic immunological features such as serum polyclonal hyper-gammaglobulinemia, positivity for multiple autoantibodies, the formation of EGCs and the potential of lymphoma development. Previous studies have shown that the numbers of CD27+ memory B cells are decreased in peripheral blood because they aggregate in inflamed salivary gland tissues in pSS patients. The accumulation of CD27+ memory B cells in salivary glands may be induced by some chemokines such as CXCR5 and CXCR4, and these cells may play a role in the formation of EGCs [15]. In contrast, CD27-negative B cells are significantly increased in peripheral blood from pSS patients. These cells may be precursors of the autoantibody-secreting cells and may be responsible for the susceptibility to lymphoma in pSS patients [15]. A novel subset of CD21–/low B cells is expanded in peripheral blood of pSS patients. As CD21 plays a central role in antigen recognition with the B cell receptor (BCR) complex, the CD21–/low B cells are anergic in response to BCR triggering. However, these cells can be activated by the Toll-like receptor (TLR) [16], suggesting a new mechanism for B cell hyperactivation in pSS.
BAFF is an important cytokine able to promote B cell proliferation, differentiation and survival [17]. However, excess BAFF may result in the accumulation of auto-reactive B cells [18]. BAFF is usually produced by monocytes, macrophages and dendritic cells in healthy people, while in pSS patients, T cells, B cells and even the salivary epithelial cells can also secrete BAFF [19]. The BAFF levels are elevated in both serum and salivary glands of pSS patients and may be correlated with the levels of autoantibodies such as anti-SSA/Ro, anti-SSB/La antibodies and rheumatoid factor (RF) [20].
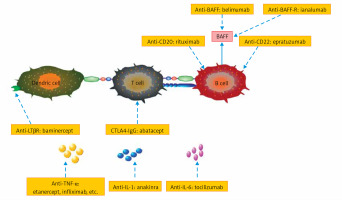
This review summarizes important and specific surface molecules in T cells and B cells, as well as critical cytokines produced by different cells. The biological agents developed on the basis of these cells and cytokines are represented in Figure 2.
Figure 2
Current targets for biologics in Sjögren’s syndrome (pSS). It is reasonable to target key cytokines or specific surface molecules on T cells and B cells for pSS treatment. The biologics in boxes will be discussed in detail below
BAFF – B-cell activating factor, BAFF-R – BAFF receptor, TNF-α – tumor necrosis factor α, LTβR – lymphotoxin β receptor, IL-1 – interleukin 1, IL-6 – interleukin 6
T cell-targeted biologic therapies
Abatacept, a fusion molecule of IgG and CTLA-4, suppresses the functions of T lymphocytes by binding to CD80/86 molecules to block the interaction of T cells with B cells as well as other antigen-presenting cells [21]. There is a paucity of data about abatacept in pSS treatment. The first prospective open-label study to test its effects on pSS showed that it inhibited glandular inflammation and decreased the total number of lymphocytic foci, with a subsequent increase in saliva production [22]. Another open-label study to assess its efficacy and safety profile (the ASAP study) demonstrated that abatacept treatment was effective, safe and well tolerated for pSS patients. ESSDAI (EULAR Sjögren’s Syndrome Disease Activity Index), ESSPRI (EULAR Sjögren’s Syndrome Patient Reported Index), RF and IgG levels were all decreased significantly after abatacept treatment [23]. The histopathological changes in parotid gland tissue after abatacept treatment were also evaluated. The number of GCs (germinal centers)/mm2 at baseline was associated with abatacept response in terms of the glandular domain of ESSDAI. Although not statistically significant, there was a clear trend showing that abatacept decreased the GC numbers [24]. Also, Verstappen et al. reported that abatacept reduced the number of circulating Tfh cells and downregulated the expression of the activation marker ICOS on T cells, which might attenuate Tfh cell-dependent B cell hyperactivity. This finding provides a working mechanism through which Tfh cells participate in the pathogenesis of pSS [21]. Currently, there are two ongoing phase III RCTs comparing abatacept with placebo (NCT02067910 and NCT02915159) to assess its efficacy and safety for pSS patients, and the results are awaited with great interest.
Another T-cell targeted biologic drug, efalizumab, has been used to treat palmoplantar psoriasis. However, it has been withdrawn from the market since April of 2009 because of severe adverse effects including multifocal leukoencephalopathy.
B cell-targeted biologic therapies
Anti-CD20: rituximab
CD20 is a molecule expressed at high levels on B cell surface and is downregulated or disappears after the terminal differentiation into plasma cells. It is associated with many physiological processes such as B cell survival, activation, proliferation and differentiation [25]. In addition, CD20 may play a role in both T cell-dependent and -independent antibody responses [26]. Moreover, it is not expressed on hematopoietic lymphoid stem cells or pre-B cells [27]. Thus, CD20 is a relatively specific and stable B cell-surface marker, and an optimal molecule for B cell depletion.
Rituximab (RTX), a chimeric murine/human monoclonal antibody (mAb) against CD20, is one of the first biologic agents used in pSS. Previous uncontrolled trials and observational studies showed a promising effect of RTX on pSS. In 2005, the result from a phase II open-label study demonstrated a satisfactory efficacy of RTX on pSS [28]. Without steroid premedication, infusions of low-dose RTX resulted in a rapid depletion of B cells in the blood and alleviated the pSS symptoms [29]. Another retrospective study revealed that RTX was effective and well tolerated in pSS patients with systemic manifestations [30]. These studies suggest that RTX is not only effective in B cell depletion but also facilitates the tapering of corticosteroid. A few subsequent studies, including open and off-label trials, have reached similar conclusions [31, 32].
Based on these results, several randomized RCTs were carried out to further evaluate the safety and efficacy of RTX for pSS. The first RCT showed significant improvement in fatigue in the RTX group compared with the placebo group on a visual analogue scale (VAS). In addition, evident improvement was observed in the social functioning score of SF-36 and in the quality of life in the RTX group to some degree at 6 months (Table I) [33]. Another RCT (Table I) showed that RTX treatment resulted in significant improvement in its primary end point of the stimulated whole saliva flow rate and some of its secondary end points (some other laboratory parameters) [34]. In this study, the stimulated saliva flow was significantly improved from 5 to 12 weeks after RTX treatment and then returned to baseline level. This finding demonstrated the efficacy of RTX in improving salivary gland function, or at least preventing it from deteriorating. A prospective, multi-center, follow-up study performed in a large cohort of early active pSS patients for a period of 120 weeks drew similar conclusions. This study compared the efficacy and safety of RTX to those of conventional DMARDs and the results showed that RTX decreased the ESSDAI scores and improved other clinical parameters in a faster and more robust manner [35]. Another small sample study of 10 patients showed an increase in the whole saliva flow rate and the lacrimal gland function [36].
Table I
Randomized controlled trials of biologic therapies for primary Sjögren’s syndrome
| Drug | Reference | Patient population | Biologic and dosage | Follow-up period | Primary end point |
|---|---|---|---|---|---|
| Rituximab | Dass et al. [33] | 17 | Two infusions of 1 g rituximab on days 1 and 15 | 6 months | > 20% improvement in fatigue VAS score (failed, but some improvement in fatigue) |
| Meijer et al. [34] | 30 | Rituximab (1,000 mg) infusions on days 1 and 15 | 48 weeks | Stimulated whole saliva flow rate (reached) | |
| Devauchelle-Pensec et al. [38] | 120 | Rituximab (1 g) infusions at weeks 0 and 2 | 24 weeks | Improvement of at least 30 mm in 2 of 4 VASs (failed, but modest efficacy for fatigue) | |
| Bowman et al. [40] | 133 | Rituximab i.v. (1000 mg in 250 ml saline) in 2 courses at weeks 0, 2, 24 and 26 | 48 weeks | Proportion of patients achieving 30% reduction in either fatigue or oral dryness VAS at 48 weeks (failed) | |
| Ianalumab (VAY736) | Dorner et al. [50] | 27 | Two different dosage: single intravenous dose ianalumab at 3 or 10 mg/kg | 24 weeks | ESSDAI (failed, but modest efficacy in most secondary endpoints in 10 mg/kg ianalumab group) |
| Infliximab | Mariette et al. [54] | 103 | Infliximab infusions (5 mg/kg) at weeks 0, 2, and 6 | 22 weeks | ≥ 30% improvement between weeks 0 and 10 in the values on 2 of the 3 VAS (failed) |
| Etanercept | Sankar et al. [56] | 28 | 25 mg of etanercept twice-weekly subcutaneous injection | 12 weeks | ≥ 20% improvement from baseline values for at least 2 of 3 domains (failed) |
| Baminercept | St Clair et al. [63] | 52 | Subcutaneous injections of 100 mg baminercept every week for 24 weeks | 24 weeks | The change between screening and week 24 in stimulated whole salivary flow (SWSF) (failed) |
| Anakinra | Norheim et al. [66] | 26 | Anakinra 100 mg/day, injected subcutaneously | 4 weeks | A group-wise comparison of the fatigue scores at week 4 (adjusted for baseline values) (failed) |
However, the observations made by different researchers are not always consistent. In an open-label clinical trial in which only 12 patients were enrolled, a single course of RTX treatment on days 1 and 15 resulted in only modest improvement in a few subjective symptoms such as fatigue and oral dryness. No significant improvement was observed in other laboratory parameters and gland function at week 26 [37]. A recent multicenter RCT conducted in France did not meet its primary end point, namely, an improvement of at least 30 mm in 2 of 4 VASs by week 24 (Table I) [38]. Rituximab seemed to rapidly induce B-cell depletion and alleviated some clinical symptoms only at an earlier time-point (at week 6), but its efficacy decreased very rapidly. Thus, the long-term efficacy of RTX for pSS treatment remained to be validated and the best interval to assess treatment efficacy in clinical trials on pSS was still uncertain. In 2014, the largest, randomized, placebo-controlled trial of RTX in patients with pSS, the Trial of Anti-B-Cell Therapy in Patients with Primary Sjögren’s Syndrome (TRACTISS), launched its protocol [39]. The most prominent difference between TRACTISS and those earlier RCTs was that patients in the RTX group were treated with two doses of the trial drug. It was expected that two courses of infusions would consolidate the long-term efficacy of RTX for pSS. However, this trial did not meet its primary endpoint or most of the secondary endpoints. The only positive finding was that patients treated with RTX maintained their baseline unstimulated salivary flow rates, while placebo patients decreased. Nevertheless, after statistical tests performed in secondary outcome analyses, the research team regarded it as a false positive finding due to a type I error (Table I) [40]. As such, the results of this largest, latest RCT did not demonstrate a beneficial effect of RTX on pSS.
To summarize, the efficacy of RTX for pSS is still controversial, but it does have an effect on fatigue, dryness and some extra-glandular manifestations, in spite of the fact that in most cases it does not seem to be cost-effective. More experiments are needed to find out which subgroup of patients is best suited for RTX.
Anti-BAFF: belimumab
As mentioned above, BAFF is a key cytokine involved in multiple physiological processes such as promotion of B cell proliferation, differentiation and survival. Belimumab, an mAb against BAFF, has shown satisfactory efficacy for SLE patients, suggesting a potential application in resistant and refractory pSS.
The Efficacy and Safety of Belimumab in Subjects with Primary Sjögren’s Syndrome (BELISS) study was the first open-label study to evaluate the efficacy and safety of belimumab in patients with pSS, and the primary endpoint was achieved in 18 (60%) patients at week 28. The ESSDAI, ESSPRI and mean dryness VAS score were all decreased significantly. In addition, the levels of most B cell biomarkers including IgG, IgA, IgM, free κ and λ light chain, mean titer of RF and the mean number of B cells were all improved [41]. At week 52, the ESSDAI and ESSPRI scores kept improving, while the decrease in biomarkers of B-cell activation observed at week 28 remained largely unchanged [42]. In 2016, the results of the follow-up after the end of the BELISS study were reported by Quartuccio and colleagues, and showed that the withdrawal of belimumab caused the relapse of pSS [43], warranting further investigations regarding its long-term efficacy for pSS. Another study showed that belimumab therapy for pSS significantly reduced transitional and naive B cell subsets and normalized BAFF-R (BAFF receptor) expression in all B subsets [44]. Further, a study focused on changes in blood lymphocyte subpopulations and labial salivary gland (LSG) inflammation after belimumab treatment in pSS patients showed a significant decrease in blood B lymphocytes (mainly CD27–/IgD+ naïve B cells) and BAFF-positive cells in foci. This study suggests that low blood and salivary NK cell numbers may serve as predictors for a better belimumab response in pSS [45]. A retrospective off-label chart review study reported that treatment with belimumab led to significantly lower epidermal growth factor (EGF) concentrations [46], although the role of EGF in the pathogenesis or development of pSS remains elusive. A large RCT is needed to solidify the abovementioned findings.
Anti-CD22: epratuzumab
CD22 is a type I transmembrane sialoglycoprotein of the immunoglobulin superfamily and can regulate B cell activation and survival via modulating BCR signaling [47]. Epratuzumab is an mAb targeting CD22 receptors on B-cells. The first open-label, phase I/II study of the effect of epratuzumab on pSS was performed in 2006. In this study, more than half of the patients achieved a clinical response (at ≥ 20% improvement level) and significant improvements in several objective and subjective parameters including fatigue and physician global assessments at different time points [48]. A recent study investigated the effect of epratuzumab on SLE-associated SS patients. While epratuzumab treatment significantly reduced the SLE disease activity, no improvements were observed in patients without SS [49]. Clearly, RCTs are needed to establish the beneficial effect of epratuzumab on secondary SS patients.
Anti-BAFF-R: ianalumab
Ianalumab (VAY736) is a human IgG1/κ mAb targeting human BAFF-R and has been investigated for its potential in pSS treatment. As such, ianalumab exerts its effect by blocking BAFF-R instead of the BAFF molecule per se. In addition, ianalumab can also eliminate B cells by antibody-dependent cellular cytotoxicity (ADCC). A single-centered, double-blind, placebo-controlled, phase II study on the safety and efficacy of ianalumab for pSS treatment is currently available. Although the primary endpoint was not met (ESSDAI score), the evaluation of almost all secondary endpoints revealed positive therapeutic effects on pSS. Patients treated with 10 mg/kg ianalumab showed an obvious and long-lasting clinical benefit, while patients treated with 3 mg/kg ianalumab only had a transient benefit [50] (Table I). A larger scale RCT with 180 patients is still ongoing and the results about the therapeutic potential of ianalumab for pSS are eagerly awaited (NCT02962895).
TNF-targeted biologic therapies
Anti TNF-α
TNF-α is one of the most important proinflammatory cytokines. Five TNF-α inhibitors are currently available for the treatment of various rheumatic diseases: etanercept, infliximab, adalimumab, certolizumab and golimumab [51]. The possible clinical benefits of TNF antagonists in pSS have been investigated for years based on the previous finding that the TNF-α level was elevated in glandular lesions of pSS patients. Infliximab, a chimeric mAb against TNF-α, was the first biologic used in pSS. A single-center, open-label pilot study including 16 patients showed a fast and significant clinical benefit, especially in alleviating fatigue, joint pain and dryness after infusions of infliximab without major adverse reactions [52]. After this study, 10 of the 16 patients continued to receive additional infusions of infliximab for 1 year and the results of the follow-up study suggested that the improvement was sustained after the additional 1-year treatment [53]. However, these two studies were retracted, partly due to the negative results of a large scale RCT, namely the TRIPSS study. In this randomized, double-blind, placebo-controlled study, these two groups (infliximab and placebo group) did not differ in either the primary endpoint or any of the secondary endpoints (Table I) [54]. Another T-cell targeted biologic drug, etanercept, is in a similar situation as infliximab. A pilot study of the TNF-α receptor antagonist etanercept demonstrated modest efficacy in a subgroup of pSS patients [55], while a subsequent RCT reported no significant differences between the etanercept and placebo group (Table I) [56]. A study in 2008 showed that TNF-α secretion was not associated with the systemic clinical parameters or the severity of pSS pathogenic lesions [57]. No more trials with other anti-TNFα biologics have been reported since then.
Anti-lymphotoxin β receptor (LTβR): baminercept
Lymphotoxin β receptor, also known as TNFRSF3, is expressed on some hematopoietic cells such as dendritic cells and tissue stromal cells such as epithelial cells. Its ligand, lymphotoxin αβ (LTαβ), is a membrane-bound heterotrimer expressed on both resting B cells and activated T cells [58]. LTβR signaling is important for secondary and ectopic/tertiary lymphoid tissue organization, a typical manifestation of B cell hyperactivation [59]. The observations obtained from different animal models indicate that the blockade of the LTβR system suppresses glandular inflammation by preventing lymphocytes from entering target tissues, and thus partially restores salivary flow [60–62]. These findings suggest that the LTβR signaling pathway is a potential target for pSS. A multicenter RCT trial was conducted in which 52 enrolled pSS patients were treated with baminercept, a LTβR fusion protein. Unfortunately, it failed to reach its primary endpoint (Table I) [63]. Although baminercept showed no improvement in the glandular signs and symptoms of pSS, this study supported the effects of LTβR signaling on the dynamics of recirculating B cell subsets and infiltrating CD4+ T cell subsets. The therapeutic benefit of LTβR signaling blockade still deserves further investigation.
Biologic therapies targeting ILs
The dysregulated cytokine network in pSS has already been reported. The pro-inflammatory cytokines are upregulated while the anti-inflammatory cytokines are downregulated in pSS patients [64]. Among all of these cytokines, IL-1 and IL-6 have been studied most extensively.
Many studies on animal models have revealed that the illness manifestations such as fatigue in pSS in humans are closely associated with IL-1 in the brain [65]. In order to examine the safety and effects of anakinra, an IL-1 receptor antagonist, a double-blind, randomized clinical trial was conducted in pSS patients. Although no significant difference was detected in its primary endpoint, more patients reported improvement in fatigue in the anakinra group compared with the placebo group (Table I) [66]. As this study was mainly focused on fatigue and depression, the assessment of the effectiveness of anakinra on pSS was incomplete. Larger scale RCTs are needed to validate its efficacy for pSS.
IL-6 is another critical pro-inflammatory cytokine involved in both B-cell and T-cell responses. An abnormally high level of IL-6 was detected in both saliva and serum in pSS patients [67]. Tocilizumab, a recombinant humanized mAb to the IL-6 receptor, has been used in different rheumatic diseases. To date, no clinical trials have been conducted in pSS patients. However, some case reports have shown some encouraging results when used for the complications associated with pSS such as refractory organizing pneumonia [68] and neuromyelitis optica [69, 70]. A randomized, double-blind, parallel, placebo-controlled trial designed to assess the efficacy of tocilizumab in pSS has started (NCT01782235) and the results are eagerly awaited.
Perspectives – future and potential new therapies for primary Sjögren’s syndrome
Many new drugs targeting cytokines and signaling pathways have been explored to find novel and effective treatments for pSS. Bortezomib, a proteasome inhibitor commonly used to treat multiple myeloma, exhibited an impressive efficacy in a severe pSS patient refractory to conventional treatment [71]. Another case report showed significant improvement after bortezomib add-on treatment in a pSS patient associated with thrombotic thrombocytopenic purpura refractory to plasma exchange [72]. Briobacept, a protein containing both IgG and the ligand binding section of the BAFF-R, exerts its effect by inhibiting BAFF. The observations made on lupus-prone mice suggest a potential application of briobacept in SLE patients [73]. It is reasonable to postulate that this drug may also be used to treat pSS. In a case of refractory pSS, the sequential belimumab-rituximab treatment seemed effective [74].
With regards to ILs, one study demonstrated elevated levels of IL-4 and IL-9 in sera of patients with connective tissue disease (CTD), especially CTD accompanied by interstitial lung disease (CTD-ILD) [75]. Based on the fact that pSS is a type of diffused CTD and ILD/pulmonary fibrosis is also common in pSS patients, IL-4 and IL-9 may be promising new targets for pSS treatment. The role of IL-17 and IL-23 in some autoimmune diseases has been investigated. It seems that IL-17 exerts a protective effect and prevents the process of autoimmunity while IL-23 may play a role in the pathogenesis of morphea [76]. The role of IL-12/23 in T cell-related chronic inflammation has been widely studied for years and blocking this pathway with ustekinumab and briakinumab have been used in some rheumatic diseases [77]. Based on the pivotal role of T cells in the initiation phase of pSS, it may be reasonable to use ustekinumab and/or briakinumab for pSS treatment, though rigorous clinical trials are still needed. The level of IL-35, a newly discovered immunoregulatory cytokine able to inhibit CD4+ T cells, is significantly lower in plasma of pSS patients, providing another interesting target for pSS treatment [78].
The role of autoantibodies against muscarinic acetylcholine receptor M3 (M3R) in the pathogenesis of pSS has been investigated. Autoantibodies against M3R have been detected in pSS patients and the presence of these autoantibodies is associated with some clinical or immunological features of pSS. However, studies from an M3R–/– mice model reveal that M3R activation is indispensable for controlling the basal exocrine secretion [79]. The endeavor to unveil the role of the phosphatidylinositol 3-kinase delta (PI3Kδ) pathway in pSS showed that this pathway was activated in human salivary gland in pSS patients as well as in a mouse model of focal sialadenitis [80]. Assessments of lymphocytic infiltration in salivary glands, the production of autoantibodies and the expression of ectopic lymphoneogenesis associated cytokines and chemokines in a mouse model after the treatment with seletalisib, an in vivo inhibitor of PI3Kδ activity, have demonstrated the therapeutic potential of blocking the PI3Kδ pathway in pSS patients.
Discussion
RTX was thought to be the most promising biologic agent to treat pSS, but it failed to meet the primary endpoint in two recent large scale RCTs. The poor sensitivity of B cells infiltrated in target organs and plasmablasts to RTX is at least partially responsible for the limited efficacy [38]. A feedback loop was observed after RTX treatment in that it caused an increase in the BAFF level in serum of pSS patients [81], leading to the development and survival of auto-reactive B cells, which may also contribute to the limited efficacy of B cell depletion therapy and the flare of pSS. This hypothesis is partially supported by the promising treatment efficacy of ianalumab [50], a new biologic agent with dual mechanisms of action: B cell depletion and blockade of BAFF-R at the same time. Thus, more studies are warranted regarding the efficacy of ianalumab and the sequential belimumab-rituximab therapy for pSS.
The dosage of biologic agents used to treat pSS patients is another issue worthy of further discussion. At present, the dosage for pSS treatment in most trials is based on that used in other autoimmune diseases with definite clinical benefit, but it may not be appropriate for pSS therapy. The insufficient drug dosage and the inability of biologic agents to enter the target organs may be responsible for the poor therapeutic effects of these biologics [56]. In addition, patients who received ianalumab in dosages of 10 mg/kg and 3 mg/kg showed different treatment responses [50]. Therefore, customized dosages of biological agents for pSS treatment are worthy of further investigation.
The adverse reactions of biological agents are another concern. Patients treated with RTX showed an increased incidence of manifestations similar to a serum sickness [34]. More infusion reactions were seen in patients receiving RTX [38]. A comprehensive review summarized a lot of previous case reports about RTX-induced serum sickness (RISS) [82].
In terms of methodology, the inconsistency of endpoints among different studies may be one of the reasons for those conflicting results. Based on previous studies on RTX, ESSDAI seems to be a good end point to assess treatment efficacy [83]. However, Cornec et al. urge that for pSS patients, the cardinal symptoms (such as dryness, fatigue, etc., best assessed by ESSPRI) are more helpful in predicting the emergence of those health-related quality-of-life impairments [84]. In fact, early in 2015, the same group suggested focusing on patients’ well-being and recommended using the SSRI (Sjögren’s Syndrome Responder Index, a composite endpoint including five outcome measures: patient-assessed VAS scores for fatigue, oral dryness and ocular dryness, unstimulated whole salivary flow and ESR) as the primary outcome measure [85]. In order to evaluate the therapeutic effect of biological agents on pSS more accurately, it is necessary to set up a reasonable, effective, concise and widely accepted outcome measure. Identical inclusion criteria may also help reduce bias and further clarify the therapeutic efficacy of the drugs studied.
In conclusion, although several biologic agents have been investigated for pSS treatment, no drug has demonstrated satisfactory efficacy. There are still many ongoing trials, and their results are awaited with great interest. More studies are needed with regards to the individualized dosages of different biologicals and reliable predictors for the good response of certain subpopulations of pSS patients to these drugs.