Introduction
In the last 50 years, obesity has become a global epidemic, with its prevalence increasing worldwide [1]. This has led the medical community to search for new treatment options alongside fundamental approaches such as dietary changes and increased physical activity. Currently, surgical treatment is the most effective weight loss method, but it comes with high risks [2]. The quest for new methods to treat obesity arises from the desire to provide treatment with lower risk and at a lower cost. The first report on the use of botulinum toxin A (BT-A) for obesity treatment was published by Rollnik et al., demonstrating a decrease in body weight and daily calorie intake after gastric BT-A injection [3]. The intragastric injection of BT-A has arisen as a potential obesity treatment due to its ability to create an early feeling of fullness [4]. Botulinum toxin is a neurotoxin produced by the Gram-positive anaerobic bacterium Clostridium botulinum [5]. It induces muscle weakness or paralysis by blocking the release of the neurotransmitter acetylcholine, which controls muscle contractions [6]. The purpose of BT-A injection is to influence gastric motility, leading to delayed gastric emptying and an earlier sensation of fullness, ultimately resulting in weight loss.
Our study aims to investigate the effects of intragastric BT-A injection on weight loss.
Material and methods
Between 2021 and 2023, a retrospective analysis was conducted on patients who underwent intragastric BT-A injection procedures in the General Surgery Endoscopy Unit. The inclusion criteria for the study consisted of being between the ages of 18 and 65 and having a body mass index (BMI) value greater than 25 kg/m2. Patients using anticoagulants, those who were pregnant or breastfeeding, those who had neuromuscular diseases, known hypersensitivity to BT-A, or cardiovascular diseases, and those with peptic ulcers that were preexisting or detected during endoscopy were not administered intragastric BT-A injections. Patients with incomplete or inaccessible hospital records were not included in the study. The weight and height measurements of the patients, as well as their BMI (weight (kg)/height squared (m2)) results, were obtained from patient records in the Endoscopy Unit before their procedures. Weight and BMI were measured at monthly intervals until the end of the study. Patient assessments were based on data at the sixth month. Appetite, early satiety, and patient satisfaction were taken into consideration. All information was obtained from the patient database and records in the endoscopy unit.
Procedure
After 8 h of fasting, patients underwent endoscopy procedures under sedation for BT-A (Botox, Allergan, Irvine, CA, USA) injection. A total of 500 units of BT-A were diluted in 12 ml of 0.9% saline solution. Injections were prepared in 12 doses, each being 1 ml. BT-A was circularly injected into the gastric wall using a standard 5-mm sclerotherapy needle. Some patients received injections covering 12 punctures near the pylorus and incisura angularis, while others received injections covering 12 punctures in the antrum and fundus regions (Figure 1). All patients received comprehensive information regarding potential side effects before the endoscopy procedure and BT-A treatment. Their medical history, including allergies and use of anticoagulant medications, was thoroughly reviewed. No acute complications were observed during the procedure. All patients were monitored for 4 h immediately after the procedure. Patients were instructed to adhere to a Mediterranean-style diet with a daily caloric intake limit of 1500 kcal. However, this dietary plan was not closely monitored or followed up.
Statistical analysis
The analyses were conducted using the IBM SPSS Statistics 22 software. Descriptive statistics are presented as n and % for categorical variables and mean ± standard deviation (mean ± SD) for continuous variables. The comparison of the categorical variables between groups was performed using the χ2 analysis method (Pearson χ2). The normality of the distribution of the continuous variables was assessed using the Kolmogorov-Smirnov test. For comparisons of two groups, Student’s t-test was used for the variables showing normal distribution, while the Mann-Whitney U test was used for the variables that did not show normal distribution. The Kruskal-Wallis test was performed for comparisons of more than two groups. The Spearman correlation test was employed to examine the relationships between non-normally distributed continuous variables. The level of statistical significance in the analyses was accepted as p < 0.05.
Results
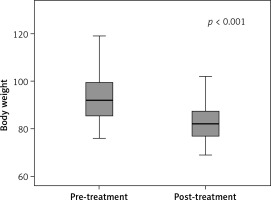
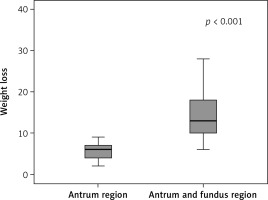
Our study included the data of 67 patients, consisting of 55 (82.1%) female patients and 12 (17.9%) male patients. The mean age of the patients was 35.5 ±9.0 years (min. = 16, max. = 58). The mean BMI of the patients was 33.5 ±3.3 kg/m², where 9% of the patients were categorized as overweight, 59.7% were in obesity class I, 23.9% were in obesity class II, and 7.5% were in obesity class III. BT-A injections were administered to 41.8% of the patients in the gastric antrum region and 58.2% of them in both the gastric antrum and fundus regions. Eleven (16.4%) patients experienced side effects, where 45.5% reported nausea, 36.4% experienced abdominal pain, 27.3% experienced constipation, 18.2% experienced headaches, 18.2% experienced flatulence, and 9.1% experienced diarrhea. Among the patients, 80.6% exhibited dietary compliance. In the assessments of the feeling of fullness among the patients, 28.4% of the patients reported a lack of satiety, 29.9% experienced mild satiety, and 41.8% reported intense satiety. Patient satisfaction levels were reported as poor by 14.9% of the patients, fair by 13.4%, good by 38.8%, and very good by 32.8% (Table I). The mean weight of the patients before treatment was 93.4 ±11.0 kg, while after treatment it decreased to 82.6 ±7.4 kg. The mean change in weight among the patients was 10.7 ±7.0 kg. A significant reduction in weight was observed following the intragastric BT-A injection and the hypocaloric diet intervention (p < 0.001) (Figure 2). The degree of weight change in the patients who received BT-A injections in both the gastric antrum and fundus regions was significantly higher than that in those who received injections in the gastric antrum region alone (p < 0.001) (Figure 3). The degree of weight change in the patients who adhered to their dietary plans was significantly greater than that in those who did not comply with their dietary plans (p < 0.001). A significant difference was observed in terms of weight change based on the feeling of fullness (p < 0.001). In the intense satiety group, weight loss was more significant. A significant relationship was observed between patient satisfaction and weight loss (p < 0.001), where patient satisfaction increased as the patients lost more weight (Table II). No significant differences were observed in the incidence of side effects based on sex, age, BMI category, or the site of intragastric BT-A injection (Table III). The intensity of feelings of satiety was significantly higher among the patients who received injections in both the gastric antrum and fundus regions (69.2%) compared to those who received injections only in the antrum region (3.6%) (p < 0.001). Furthermore, the dietary adherence rate was significantly higher among those who received injections in both the antrum and fundus regions (100%) compared to those who received injections only in the antrum region (53.6%) (p < 0.001). The satisfaction rate for those who received injections in both the antrum and fundus regions (53.8%) was significantly higher than in those who received injections only in the antrum region (3.6%) (p < 0.001) (Table IV).
Table I
Patient characteristics
| Parameter | N | % |
|---|---|---|
| Gender | ||
| Female | 55 | 82.1 |
| Male | 12 | 17.9 |
| Age, mean ± SD | 35.5 ±9.0 | |
| BMI, mean ± SD | 33.5 ±3.3 | |
| BMI categories | ||
| Overweight | 6 | 9.0 |
| Obesity class I | 40 | 59.7 |
| Obesity class II | 16 | 23.9 |
| Obesity class III | 5 | 7.5 |
| Injection site | ||
| Antrum | 28 | 41.8 |
| Antrum-fundus | 39 | 58.2 |
| Presence of side effects | ||
| Yes | 11 | 16.4 |
| No | 56 | 83.6 |
| Type of side effects* | ||
| Nausea | 5 | 45.5 |
| Abdominal pain | 4 | 36.4 |
| Constipation | 3 | 27.3 |
| Headache | 2 | 18.2 |
| Flatulence | 2 | 18.2 |
| Diarrhea | 1 | 9.1 |
| Dietary compliance | ||
| Yes | 54 | 80.6 |
| No | 13 | 19.4 |
| Satiety feeling | ||
| Lack | 19 | 28.4 |
| Mild | 20 | 29.9 |
| Intense | 28 | 41.8 |
| Patient satisfaction | ||
| Poor | 10 | 14.9 |
| Fair | 9 | 13.4 |
| Good | 26 | 38.8 |
| Satisfied | 22 | 32.8 |
Table II
Comparison of weight difference according to various parameters
| Parameter | Weight difference | |
|---|---|---|
| Mean ± SD | P-value* | |
| Gender | ||
| Female | 10.0 ±5.7 | 0.276 |
| Male | 14.1 ±10.7 | |
| BMI categories | ||
| Overweight | 6.7 ±3.3a | 0.039** |
| Obesity class I | 9.3 ±5.3a | |
| Obesity class II | 13.8 ±9.2a.b | |
| Obesity class III | 17.4 ±7.8b | |
| Injection site | ||
| Antrum | 5.6 ±2.1 | < 0.001 |
| Antrum-fundus | 14.4 ±6.9 | |
| Presence of side effects | ||
| Yes | 11.2 ±6.9 | 0.919 |
| No | 10.7 ±7.0 | |
| Dietary compliance | ||
| Yes | 12.4 ±6.8 | < 0.001 |
| No | 4.1 ±1.8 | |
| Satiety feeling | ||
| Lack | 5.6 ±2.1a | < 0.001 |
| Mild | 7.5 ±3.0a | |
| Intense | 16.5 ±6.9b | |
| Patient satisfaction | ||
| Poor | 4.2 ±1.5a | < 0.001 |
| Fair | 5.3 ±2.2a | |
| Good | 9.7 ±3.5b | |
| Satisfied | 17.2 ±7.5c | |
Table III
Comparison of adverse effects according to various parameters
| Parameter | Adverse effects present | Adverse effects absent | P-value* | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | |||||
| Female | 8 | 14.5 | 47 | 85.5 | 0.400 |
| Male | 3 | 25.0 | 9 | 75.0 | |
| Age, mean ± SD | 38.1 ±6.9 | 35.0 ±9.3 | 0.293** | ||
| BMI categories | |||||
| Overweight | 0 | .0 | 6 | 100.0 | 0.552 |
| Obesity class I | 7 | 17.5 | 33 | 82.5 | |
| Obesity class II | 4 | 25.0 | 12 | 75.0 | |
| Obesity class III | 0 | .0 | 5 | 100.0 | |
| Injection site | |||||
| Antrum | 2 | 7.1 | 26 | 92.9 | 0.104 |
| Antrum-fundus | 9 | 23.1 | 30 | 76.9 | |
Table IV
Comparison of satiety feeling, dietary compliance, and satisfaction status by injection site
| Parameter | Antrum | Antrum-fundus | P-value* | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Satiety feeling | |||||
| Lack | 16 | 57.1 | 3 | 7.7 | < 0.001 |
| Mild | 11 | 39.3 | 9 | 23.1 | |
| Intense | 1 | 3.6 | 27 | 69.2 | |
| Dietary compliance | |||||
| Yes | 15 | 53.6 | 39 | 100.0 | < 0.001 |
| No | 13 | 46.4 | 0 | 0.0 | |
| Patient satisfaction | |||||
| Poor | 10 | 35.7 | 0 | 0.0 | < 0.001 |
| Fair | 7 | 25.0 | 2 | 5.1 | |
| Good | 10 | 35.7 | 16 | 41.0 | |
| Satisfied | 1 | 3.6 | 21 | 53.8 | |
Discussion
The data of a total of 67 patients were included in the study. At the 6th-month follow-ups after intragastric BT-A applications, the patients had a mean weight loss of 10.7 ±7.0 kg. This result was similar to the 2008 report by Foschi et al. [7]. Their study, which was pioneering in terms of its inclusion of the gastric fundus, produced results similar to ours, indicating that intragastric BT-A injection leads to substantial weight loss in obese patients. The fundamental reason for using intragastric BT-A in obese patients is its effectiveness in slowing gastric motility, resulting in early satiety and contributing to weight loss [8]. In our study, 20 out of the 67 patients experienced a mild decrease in appetite after intragastric BT-A injection, while 28 patients experienced a significant decrease in appetite. Additionally, our study showed a significant difference in weight change based on satiety (p < 0.001). Patients who adhered to their diet plans lost significantly more weight than those who did not (p < 0.001). Our study also revealed a significant relationship between patient satisfaction and weight loss (p < 0.001). It was also found that patient satisfaction increased in proportion to weight loss. This study demonstrated the clear effect of intragastric BT-A injection in combination with diet, resulting in a mean weight loss of 10.7 ±7.0 kg. Bang et al. concluded in their meta-analysis that the intragastric injection of BT-A resulted in weight loss, and a larger injection area, including the fundus or proximal body, was associated with a greater degree of weight loss [9]. Similarly, Karaca observed a significant trend of increasing weight loss over a 3-month follow-up period following the injection of 400 IU BT-A into the gastric mucosa of the antrum, corpus, and fundus regions [10]. In our study, we compared the degree of weight loss between patients who received BT-A injections in both the antrum and fundus regions and those who received injections only in the gastric antrum region. Our results indicated that the degree of weight loss observed in the patients who received injections in both the gastric antrum and fundus regions was significantly greater than that in the patients who received injections only in the gastric antrum region (p < 0.001). Additionally, it was suggested that BT-A injections in the fundus may play an important role in disrupting gastric accommodation, inhibiting ghrelin secretion, and consequently increasing the feeling of satiety [11, 12]. Similarly, we observed that the rate of reporting a high intensity of satiety in the patients who received BT-A injections in both the antrum and fundus regions was significantly higher than that in those who received injections only in the antrum region (p < 0.001).
In 2020, Ferhatoğlu et al. found that combining a high-protein hypocaloric diet with BT-A administration produced better results than the sole usage of either the diet or BT-A [13]. Although a hypocaloric diet was prescribed to all patients in our study, we noticed during our follow-ups that some patients did not adhere to their dietary plans. As in the study by Ferhatoğlu et al., we found that the patients who adhered to the dietary guidelines prescribed to them had a significantly higher degree of weight loss than those who did not (p < 0.001). In contrast, in the study carried out by Kaya et al., although a low-calorie diet was recommended to all patients after the procedure, there was no statistically significant difference in weight loss between the patients who followed the dietary plan and those who did not [14]. Topazian et al. [15] concluded that BT-A application was not associated with early satiety, changes in eating behaviors, or weight loss, but it delayed gastric emptying at a dose of 300 IU. On the other hand, García-Compean et al. reported that BT-A injections in the antral region did not significantly reduce weight or delay gastric emptying in obese patients [16]. Nevertheless, in our study, the patients who received BT-A injections, especially those who received injections in both the antrum and fundus regions and followed a compatible diet, experienced significant weight loss.
Albani et al. observed a total weight loss of approximately 4 kg over a 1-month follow-up period by administering 500 IU of BT-A to a small group of patients [17]. In our study, we also administered 500 IU of BT-A to each patient. However, it is worth noting that many studies have shown that the efficacy of BT-A may not be dose-dependent, and a dose as low as 200 IU showed positive results in terms of weight loss [7, 18].
Chang et al. suggested that intragastric BT-A injection was not more effective than saline injection in the control group regarding absolute weight loss or change in BMI, but it significantly prolonged the time of gastric emptying [19]. The recent meta-analysis by Bustamante et al. revealed that BT-A alone was ineffective as a primary treatment for obesity [20]. On the other hand, Hsu et al. observed that intragastric BT-A injection combined with dietary control resulted in 11.5% greater weight loss than in other randomized controlled trials. They attributed these results to the injection of a higher dose of BT-A (300 IU) into the gastric fundus, body, and antrum, leading to increased satiety and prolonged gastric emptying times [21]. There is no consensus on the method of BT-A injection in terms of the number of injections or puncture sites, and there is still no agreement on the exact effects of BT-A in the treatment of obesity. However, the results of our study demonstrated that intragastric BT-A injection, including antrum and fundus injections, was an effective and safe procedure for achieving satiety and moderate weight loss. One of the main advantages of the intragastric injection of BT-A is the low likelihood of serious side effects. In our study, although side effects such as nausea, abdominal pain, constipation, headache, flatulence, and diarrhea were observed, there were no severe complications during or after the procedure.
Smoking, obesity, and low physical activity are the primary negative lifestyle factors that require improvement in patients [22]. In obesity treatment, both current and future interventions are personalized according to patient needs. Current approaches include dietary changes, regular physical activity, behavioral therapy, pharmacotherapy, and bariatric surgery. Hao et al. suggest that combining resistance training with moderate to high-intensity aerobic exercise is the most effective approach for treating primary obesity in adolescents [23]. BT-A injections in obesity management are an emerging intervention, not yet widely established. Current indications involve experimental use, primarily in clinical trials or controlled settings. This procedure may be considered for obese patients who have not responded adequately to conventional treatments such as diet, exercise, and behavior modification. Prospective indications might expand as more research is conducted, potentially including a wider range of obese patients, particularly those who are not ideal candidates for bariatric surgery. Additionally, SGLT-2 inhibitors (SGLT2is) and GLP-1 receptor agonists (GLP-1RAs) have become important in managing type 2 diabetes and obesity due to their distinct mechanisms of action and clinical benefits. SGLT2is help to improve insulin secretion without raising insulin levels and aid in weight loss through osmotic diuresis and calorie excretion in urine. GLP-1RAs delay gastric emptying and directly affect the central nervous system to suppress appetite, promoting weight loss. Both drug classes exhibit significant clinical benefits, including improved glycemic control, systolic blood pressure reduction, weight loss, and dyslipidemia management. In summary, both SGLT2is and GLP-1RAs are valuable for managing type 2 diabetes, with additional benefits for cardiovascular health and weight management [24, 25]. However, choosing the right intervention based on individual patient profiles and treatment goals is essential. Future obesity treatments may shift towards more personalized approaches based on genetic and metabolic analyses. In particular, genes in the leptin-melanocortin signaling pathway and their role in energy balance and body weight regulation could significantly contribute to these personalized treatments. Research in polygenic obesity, showing the interaction of multiple genes with environmental factors leading to obesity, will play a crucial role in developing future treatments [26].
Our study had several limitations. Firstly, it was a retrospective, single-center study. Secondly, the study included a small number of patients, which may limit the generalizability of the findings. Thirdly, we did not investigate factors such as exercise or medication use, which could have influenced the outcomes of weight loss. Additionally, the study lacked comprehensive dietary monitoring. Lastly, the absence of a control group should also be noted as a limitation.
In conclusion, this study demonstrates the potential benefit of intragastric BT-A injection for weight loss. This procedure is a minimally invasive and cost-effective option with a low risk of side effects. However, it should be stated that some patients in our study did not experience weight loss or decreased appetite. Furthermore, there were differences in outcomes between patients who followed dietary guidelines and those who did not. Therefore, further large-scale, randomized, and prospective studies are needed to understand the efficacy of the intragastric injection of BT-A.