We present a case report of 38-year-old female with aneurysmal subarachnoid hemorrhage (aSAH), who underwent successful endovascular coiling of aneurysms of the right middle cerebral artery (MCA) and the anterior communicating artery (Acom) in our high-volume aSAH (> 35 cases per year) medical centre. The patient initially presented the typical signs and symptoms of SAH: severe headache, vertigo, nausea, vomiting, high arterial blood pressure (ABP) (180/100 mm Hg). There was a history of arterial hypertension, which is also a common risk factor for cerebral aneurysm formation and subsequent rupture. Following the endovascular procedure, the patient was admitted to the local intensive care unit (ICU) for close monitoring and management of neurologic status and systemic physiology. Following the percutaneous embolization, the patient was fully conscious, with logical verbal contact, oriented to the person/time/location; pupils were narrow, equal, reactive; eyeballs pointed towards the examiner; no meningeal signs were present. The management of the patient followed the most recent neurocritical care guidelines for aSAH patients [1, 2]. Neurologic monitoring of the patient involved regular clinical assessment (at least every 2 h) of the neurologic status, aimed particularly at the level of consciousness, intensity of headache and vertigo, presence of nausea and vomiting, and the presence of local neurologic deficits. Neurologic status monitoring involved also diagnostic imaging: computed tomography (CT), computed tomography angiography (CTA), digital subtraction angiography (DSA), and point-of care transcranial Doppler (TCD) ultrasonography (US) with transcranial color-coded duplex (TCCD). The diagnostic imaging was used for timely detection of typical neurologic complications of aSAH such as cerebral oedema, re-bleeding, hydrocephalus, vasospasm (VS), and delayed cerebral ischemia (DCI). Systemic physiology monitoring included peripheral oxygen saturation (SpO2), respiratory rate, continuous electrocardiography, invasive arterial blood pressure (IABP) monitoring, serial echocardiographic examination, serial US examination of diameter and collapsibility of inferior vena cava, temperature, glucose concentration, and acid-base and electrolyte balance. Systemic physiology management involved keeping mean arterial pressure (MAP) at ≥ 90 mm Hg (IABP transducer kept at the level of external auditory meatus), ensuring euvolaemia, normal core temperature, arterial blood glucose concentration ≤ 180 mg/dl, serum sodium in the upper range and other electrolyte concentrations within reference range, and normal acid-base balance. The pharmacological therapy of the patient included oral nimodipine 60 mg every 4 h, oral acetylacetic acid 150 mg once daily, subcutaneous enoxaparin 60 mg twice daily, paracetamol, metamizole, pregabalin, oxycodone, unbalanced (0.9% sodium chloride) and balanced crystalloid (Plasmalyte, Baxter, Poland) according to fluid responsiveness, and oral alimentary formula (Nutridrink, Nutricia, Poland). The patient was nurtured in a 30° head-of-bed elevated position.
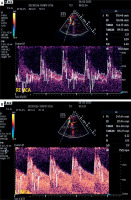
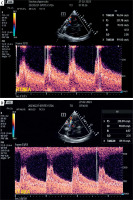
Despite maximal pharmacological prophylaxis with oral nimodipine, maintaining euvolaemia and appropriate MAP, the patient developed bilateral MCA VS, which was initially detected by TCCD examination (M7, Mindray, Peoples Republic of China) on day 10 post ictus with mean cerebral blood flow velocity (mCBFV) 119.5 and 121.3 cm/s on the right and left MCA, respectively (Figures 1 A, B) (26.02.2023). The Lindegaard index (LI) was 7.3 and 7.1 on the right and left side, respectively. The presence of bilateral VS was confirmed by CTA examination. The patient complained of headaches with varying degrees of intensity (up to 9 on a Numerical Rating Scale) only partially responding to analgesics, vertigo, and nausea. The patient had a variable degree of consciousness, from full consciousness though disorientation to somnolence. The initial approach to VS management was graded arterial blood hypertension with acceptance of supra-normal pressures up to 200 mm Hg systolic, switch from oral to intravenous nimodipine (infusion 2 mg/h), and introduction of rosuvastatin (40 mg once daily). These attempts at alleviating VS were unsuccessful; therefore, interventional neuroradiology consultation was requested and the patient was scheduled for endovascular rescue treatment – administration of intraarterial nimodipine. During the procedure, nimodipine (Nimotop S, Bayer AG, Germany) in a 1 mg bolus given over 10 min was deposited in the M1 segment of the MCA bilaterally. The TCCD examination of MCAs following the procedure is shown in Figures 1 C, D. The patient underwent a series of 6 unilateral or bilateral intraarterial injections of nimodipine depending on the anatomy of vasospasm. The median MCA peak, diastolic, and mean CBFVs before and after procedure were 217.3 (IQR: 201.3–281.4) vs. 136.7 (IQR: 96.2–173.5), 91.4 (IQR: 80.9–127.2) vs. 58.7 (IQR: 43.1–82.7), and 92.3 (IQR: 74.8–103.4) vs. 55.5 (IQR: 41.9–61.7) cm/s, respectively (p for all < 0.01). The median decreases of these CBFVs were 103.7 (IQR: 63.5–121.4), 44.7 (IQR: 25.7–81.3), and 28.2 (IQR: 24.9–39.6) cm/s, respectively. Despite numerous intraarterial injections of nimodipine, the patient developed DCI involving multiple brain regions. In neurological examination at the discharge from the ICU the patient was conscious, auto- and partly allo-psychic oriented, with communication impeded due to tracheostomy, no meningeal symptoms, a tendency to rotate the eyes to the right, left-sided amblyopia, paresis of the right lower limb III degree, left upper limb and lower grade I, spastic increased muscle tone in all limbs, exaggerated tendon reflexes, and positive Babinski sign on both sides. The patient was transferred to the neurological rehabilitation department.
Figure 1
Transcranial color-coded duplex examination of right (A) and left (B) middle cerebral artery before and after (C, D) bilateral intra-arterial nimodipine administration
Aneurysmal subarachnoid haemorrhage (aSAH) is an acute cerebrovascular event with the potential to cause devastating injury to the central nervous system (CNS) and have a deleterious impact on distant organs. It is a disease that is associated with high mortality and often very poor prognosis for functional recovery in survivors. Neurologic complications frequently lead to secondary brain injury in these patients. Non-neurological complications are also frequent following SAH, and early recognition and prompt intervention might improve outcome [3]. Due to complicated clinical course, all aSAH patients should be transferred to high-volume centres and hospitalized in an intensive care unit (ICU) with appropriate experience and expertise [1, 2]. The complex management of patients following SAH is also the reason for listing SAH as a typical indication for ICU hospitalization.
The presented case shows refractory bilateral VS of MCA following successful coiling of a ruptured aneurysm of the right MCA and AComA. Vasospasm is an arterial narrowing after SAH detected by radiographic or ultrasonography imaging [4]. Vasospasm may occur after 2–17 days post ictus, timing depending on the severity of aSAH, with higher-severity SAH associated with earlier incidence of VS. Vasospasm is triggered by the breakdown of blood constituents that accumulate in the subarachnoid and perivascular space, in particular oxyhaemoglobin. Extravasated oxyhaemoglobin may lead to direct vasoconstriction, release of arachidonic metabolites and endothelin from the arterial wall, inhibition of endothelium-dependent vasodilation by scavenging nitric oxide, damage to perivascular nerves, and promotion of free radical reactions [5]. Numerous other factors may contribute to the development DCI following SAH, including but not limited to distal microcirculatory failure, poor collateral anatomy, and genetic or physiological variations in ischaemic tolerance [6]. All SAH patients should receive enteral nimodipine, a specific antagonist of L-type voltage-gated calcium channels, 60 mg four-hourly immediately after diagnosis. This reduces the incidence of DCI and improves outcome [7]. TCD should be considered for the visualisation of VS in patients following aSAH [2]. Different TCCD criteria are used for defining VS in different vascular regions. The criteria for proximal MCA VS were described as follows: moderate VS (mCBFV ≥ 120 cm/s; LI 5-6), severe VS (mCBFV ≥ 200 cm/s; LI > 6) [8].
Despite accepting supra-normal arterial blood pressures, continuing maximal standard pharmacological therapy and introduction of additional pharmacological agent (rosuvastatin), bilateral MCA VS persisted. A large randomized controlled trial showed no benefit of 40 mg simvastatin in terms of short-term and long-term outcome [9]. Nevertheless, we decided to use statin due to the lack of safety concerns presented in the aforementioned trial. In patients with aSAH undergoing clipping of the aneurysm through craniotomy, drainage of cerebrospinal fluid from the carotid cistern may remove the subarachnoid blood and reduce the risk of VS [10]; however, in our patient percutaneous intravascular embolization was performed. Intraarterial administration of vasodilators may be used when standard medical therapy is not effective [2]. Small clinical studies show that intraarterial administration of nimodipine might have a beneficial effect on functional and neuropsychological outcomes [11]. Therefore, an attempt at reversing VS with intraarterial administration of nimodipine was made. Due to the anatomy of the VS the angioplasty was no therapeutic option in this patient. Despite hospitalization in the ICU with close monitoring of neurologic status and systemic physiology, prompt management of derangements and the use of all treatment modalities recommended in the appropriate guidelines including rescue therapy by an experienced interventional radiologist, the patient developed DCI in multiple brain regions. The possible reason for the rescue therapy failure in our case could be the transient effect of nimodipine injected intraarterially. To overcome this impediment continuous intraarterial nimodipine infusion could be used [12]. The presented case shows that a single rescue therapy for VS may not be effective; however, the decision regarding introduction of additional rescue therapies may be difficult as to the timing. Nevertheless the neurologic status of the patient could have been worse if the patient was not hospitalized in the ICU in our aSAH high-volume centre with expertise in treating aSAH patients and access to VS rescue treatment modalities.
In conclusion, intraarterial administration of nimodipine may not be effective as a rescue therapy for refractory VS following aSAH. In the case of intermittent intraarterial nimodipine administration failure, continuous intraarterial nimodipine infusion or cerebral angioplasty could be explored; however, the optimal timing of additional rescue therapies remains unresolved. TCCD monitoring is a useful method for early detection and monitoring of VS. Patients with aSAH should be transferred to high-volume centres because significant expertise and access to VS rescue therapies is required for optimal management of aSAH patients for the best possible neurological recovery.