Introduction
Insulin-like growth factors (IGFs), also called somatomedins, are synthesized under the influence of growth hormone (GH) and form with it the somatotropin axis, which plays a key role in the regulation of growth-related processes. So far, two somatomedins have been discovered: IGF-1 and IGF-2. According to biochemical background they belong to the proteins related to insulin [1]. Both IGFs are composed of single polypeptide chains structurally similar to insulin [2, 3]. IGF-1 consists of 70 amino acids, while IGF-2 is 67 amino acids long and has a 65% homology to IGF-1 [4]. They show cytophysiological activities, i.e., activation of DNA replication and RNA transcription, protein synthesis (e.g., collagen and proteoglycans), regulation of cell division and growth (e.g., chondroblasts and chondrocytes).
Insulin-like growth factor-1 influences the absorption of sulfates into cartilage and bone, bone mineralization and an increase in the kidney of the process of phosphate reabsorption, influencing mainly bone growth in terms of length [5], whereas IGF-2 shows a strong (stronger than IGF-1) mitogenic effect mainly on muscle cells, influencing their differentiation and maturation [5, 6].
Since IGFs have a structure similar to insulin, they also exert comparable effects through insulin receptors. However, they are 16 times weaker. IGFs via the insulin receptor lead to inhibition of lipolysis, increased transport of glucose to adipocytes, glucose oxidation, and a reduction in the release of free fatty acids [2]. It has also been confirmed that insulin deficiency itself is associated with decreased IGF-1 values [7].
Insulin-like growth factors are mainly synthesized in the liver. In addition, they can be found in fibroblasts, myoblasts, chondroblasts, osteoblasts, brain cells, gastrointestinal epithelium, kidneys and adipose tissue. Their production depends on many factors, such as age, sex, circadian rhythm, genetic factors and the presence of chronic diseases [7, 8]. In chronic liver disease, the IGF-1 production is reduced, while chronic renal failure leads to a decrease in the bioavailability of this molecule, despite its normal or sometimes elevated levels [9–11].
A major observation of researchers was finding a correlation and a significant influence of reduced IGF-1 concentrations on the development of cardiovascular diseases. Also a negative correlation has been documented between somatomedin and fibrinogen and homocysteine concentrations, which are independent coronary heart disease and stroke risk factors [12–15]. Patients with chronic, impaired IGF-1 production also show endothelial dysfunction and premature atherosclerosis, and they suffer from serious vascular clinical events, such as heart attack or stroke [16–18].
Previous studies in heart failure (HF) patients have assessed mainly IGF-1. An overwhelming number of researchers have mostly observed a significant reduced IGF-1 concentration in HF. No previous studies have assessed the IGF-2 in patients with chronic HF despite reports indicating its stronger mitogenic effect on cardiomyocytes. We decided to evaluate the IGF-1 and IGF-2 concentrations depending on the severity of HF and annual mortality.
Material and methods
Methods
The study included patients consecutively admitted to the Department of Cardiology, Multispecialty Hospital in Inowroclaw with newly diagnosed or worsening HF. The exclusion criteria were a history of active neoplastic disease (< 5 years), acute HF requiring catecholamine treatment, acute infection, advanced chronic obstructive pulmonary disease, renal and liver dysfunction (creatinine concentration over 2 mg/dl and transaminase 2–3 times above normal) and mental illness. The condition for participation in the study was signing the informed consent form.
Each participant was examined with the following measurements during the admission to the hospital: height, weight, waist and hip circumference and blood pressure; blood collection for routine/base laboratory tests including the natriuretic peptide (BNP) and glycated hemoglobin (HbA1c) concentrations. During the blood collection for routine laboratory tests, 2 ml of peripheral venous blood was additionally collected for IGF-1 and IGF-2 determinations. During the first 48 h after admission all patients underwent echocardiographic examination (echo) in accordance with the guidelines of the Echocardiography section of the Polish Cardiac Society [19].
All routine laboratory tests and determination of BNP and HbA1c concentrations were performed at the Central Laboratory of Multispecialist Hospital in Inowrocław. BNP concentration was measured with the Human Brain Natriuretic Peptide ELISA kit from Sigma-Aldrich and HbA1c with the Human Glycated Hemoglobin A1c ELISA kit from MyBioSource, using the Sysmex XN-1000 automated analyzer. IGF-1 concentration was determined by enzyme immunoassay using IGF-1 600 ELISA from DRG, and IGF-2 was assessed using IGF-II Human ELISA from BioVendor. Both parameters were measured at the Department of Laboratory Medicine, Collegium Medicum UMK in Bydgoszcz.
The transthoracic echo examination was performed at rest, in the lying position on the left side with calm breathing pattern in one-dimensional and two-dimensional presentation, using the Doppler pulsation method and the Vivid 7 pro equipped with a mechanical probe (2.5 MHz frequency). The image of the heart was obtained in the following views: the parasternal in the long axis of the left ventricle, the parasternal in the short axis of the left ventricle, and the apical four-chamber projection. The following echocardiographic parameters were analyzed: the left atrium (LA) size, the left ventricle (LV) size, and the ejection fraction (EF) of the left ventricle using the biplane Simpson method.
One year after hospitalization, participants or their designated representatives were contacted by phone to obtain information on survival. The causes of deaths were verified based on the provided medical documentation.
Patients
The study included 75 patients (64% men). The mean (SD) age of researched subjects was 67.11 (13.56) years, body mass index (BMI) was 30.18 (6.17) kg/m2 and waist-to-hip ratio (WHR) was 0.99 (0.11). Most of the patients had excessive body weight: 41% were overweight, 43% were obese. Forty-two patients (56% of all subjects) were diagnosed with carbohydrate metabolism disorders before hospitalization (51% with type 2 diabetes, and 5% with prediabetes). The mean (interquartile range) HbA1c was 44.0 mmol/mol (40.0–51.0), EF: 41% (26–55) and BNP 517.27 pg/ml (235.41–1566.34). Ischemic HF etiology was found among 43% of patients, due to arrhythmia in 41%, and due to valvular defects in 16% of patients. In the research group, 26 (34.7%) patients presented HF with preserved ejection fraction, 13 (17.3%) presented HF with midrange ejection fraction and 36 (48%) had HF with reduced ejection fraction [20].
All participants have been classified according to functional class II to IV in addition to the New York Heart Association (NYHA) classification. The subjects were divided into two groups depending on the severity of HF. One group enrolled the patients with NYHA class II, while the second included patients with classes III and IV. Characteristics of the study group according to the NYHA classification are presented in Table I. Patients from the two groups did not differ in age, sex, BMI and WHR indexes, glycemia, HbA1c, creatinine, high-density lipoprotein (HDL) and triglyceride concentrations or glomerular filtration rate (eGFR). However, they differed significantly in echocardiographic parameters, BNP, total cholesterol (TC) and low-density lipoprotein (LDL) concentrations. NYHA II patients presented significantly smaller LA and LV dimensions and also had a significantly higher EF value. NYHA III/IV patients had a significantly higher BNP level and lower TC and LDL concentrations compared to NYHA II patients, despite the fact that they were treated with statin significantly less frequently and therapy with an aldosterone receptor antagonist was introduced more regularly for this group (Table I).
Table I
Characteristics of the studied group according to the NYHA classification
[i] NYHA – the New York Heart Association, BMI – body mass index, WHR – waist-to-hip ratio, LA – left atrium size, LV – left ventricle size, EF – ejection fraction of the left ventricle, LDL – low-density lipoprotein, HDL – high-density lipoprotein, BNP – natriuretic peptide, HBA1c – glycated hemoglobin, ACE-I – angiotensin convertase inhibitor, ARB – angiotensin receptor blocker, ASA – acetylsalicylic acid, SD – standard deviation, IQR – interquartile range.
Nine (12%) patients died during the 12-month follow-up. All deaths were due to cardiovascular causes.
Statistical analysis
The summary statistics are presented as the mean and standard deviation (SD) for normally distributed continuous variables and as the median with interquartile range (IQR) for non-normally distributed variables. Categorical variables are presented as frequencies.
Differences between continuous normally distributed variables were analyzed by the t test for independent samples or by ANOVA together with the adjustment for multiple testing. If the data were not normally distributed, differences were tested by the Wilcoxon and Kruskal-Wallis test. When multiple patient groups were compared, multiple testing corrections were also applied. Differences for categorical variables were tested using the χ2 or Fisher exact test for independence. Pearson’s r correlation coefficient was used to test the strength of the correlation between the selected continuous-type variables. To identify independent death risk factors a statistical regression model was used.
The results were considered as statistically significant when the p-value was less than 0.05. The statistical analysis was performed using the R software, version 3.0.3.
Results
We found that IGF-1 concentration was not statistically significantly different among patients with various HF severity according to the NYHA classification, BMI value and presence of carbohydrate metabolism disorders. It was also not different in the surviving patients compared to the patients who died during the 12-month follow-up.
There were also no statistically significant differences in IGF-2 concentrations depending on BMI value and the presence of carbohydrate metabolism disorders. The group of patients with more advanced HF (NYHA III/IV) showed a significantly lower IGF-2 concentration compared to NYHA II patients. Also, lower values of somatomedin were characteristic for people who died during the 12-month follow-up (Table II).
Table II
IGF-1 and IGF-2 concentration in the study groups
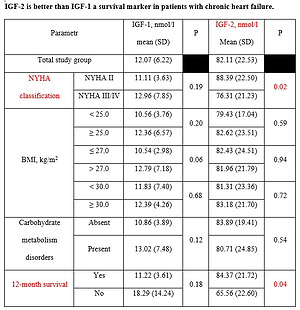
We assessed the correlations between IGF-1 and IGF-2 concentrations and age, gender, BMI and WHR index, heart rate in the ECG record, echocardiographic parameters, i.e., LA, LV, and EF as well as the glycemia, creatinine, TC, HDL, LDL, triglycerides, BNP and HbA1c concentrations. There were no significant correlations for IGF-1. We observed a positive correlation between IGF-2 and TC concentrations (r = 0.28, p = 0.01) and the LDL fraction concentration (r = 0.29, p = 0.011) (Figure 1). We found similar correlations in the NYHA III/IV group patients only. In this group IGF-2 positively correlated with concentrations of TC (r = 0.42, p = 0.008), LDL (r = 0.43, p = 0.007) and triglycerides (r = 0.35, p = 0.031) (Figure 2). We did not find such correlations in NYHA II patients.
Figure 1
Correlation of IGF-2 concentrations with TC (A) and LDL (B) concentrations
IGF-2 – insulin-like growth factor type 2, LDL – low-density lipoprotein, TC – total cholesterol.
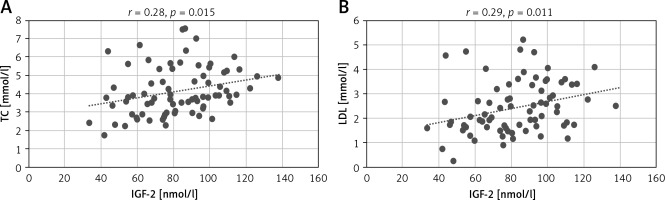
Figure 2
Correlations of IGF-2 concentrations with TC (A), LDL (B), and TG (C) concentrations
IGF-2 – insulin-like growth factor type 2, LDL – low-density lipoprotein, TC – total cholesterol, TG – triglycerides.
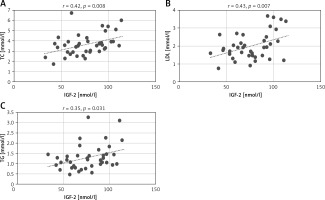
In the groups divided depending on BMI, among patients with BMI ≥ 25 kg/m2, IGF-2 concentrations correlated positively with TC (r = 0.31, p = 0.014) and LDL level (r = 0.28, p = 0.028) (Figure 3). There were no significant correlations of IGF-1 concentration in these groups.
Figure 3
Correlation of IGF-2 concentrations with TC (A) and LDL (B) concentrations
IGF-2 – insulin-like growth factor type 2, LDL – low-density lipoprotein, TC – total cholesterol.
To identify independent annual death risk factors a regression model was used. Table III presents the estimation results of the models with a single explanatory variable (Model I) and with the multivariable model (Model II). The concentrations of IGF-2 and HbA1c as well as the LA size had an impact on the mortality of patients with chronic HF during the 12-month follow-up.
Discussion
In HF patients the vast majority of researchers have so far observed GH deficiency and decrease of IGF-1 concentrations [21, 22]. Jankowska et al. noted reduced IGF-1 values in 64% of HF patients [23]. In this group of patients, it was found that low IGF-1 concentration correlated positively with the degree of systolic dysfunction, the presence of cachexia and skeletal muscle weakness, as well as interacting with neurohormonal activation (synthesis of cortisol and natriuretic peptides) and proinflammatory cytokines [24, 25]. Some researchers have found that low IGF-1 concentration mainly affected patients with cardiac cachexia. Anker et al. did not find a decreased IGF-1 concentration in HF patients without cachexia; however, along with the symptoms of malnutrition, the values of this protein decreased significantly [26]. Research by Petretta et al. indicated that low IGF-1 values in relation to GH with simultaneous high NT-proBNP concentration may be considered as independent death predictors in HF patients without cachexia [27].
Experimental studies explaining the mechanism of the beneficial effect of IGF-1 on the heart muscle function and patient survival indicated the influence of this somatomedin on inhibition of cardiomyocyte apoptosis. This was documented by activating the PI3k/Akt (phosphatidylinositol 3-kinase and AKT protein kinase) signaling pathway and feedback inhibition of the SOCS (suppressors of cytokine signaling) pathway, which are the mechanisms of apoptosis [28, 29]. Repetto et al. stated that IGF-1 also participates in proper functioning of the vascular endothelium by stimulating nitric oxide production, thus exerting an antiatherosclerotic effect [30], and it may explain the beneficial effect of the protein on cardiomyocyte function and, consequently, patient survival.
In our study, however, we did not find significant differences in IGF-1 levels depending on HF severity according to the NYHA classification, but we observed statistically significantly lower IGF-2 levels in patients with advanced (NYHA III/IV) HF compared to patients with NYHA II.
Most of the existing studies focus only on IGF-1, while data on IGF-2 in HF patients are insufficient. The influence of IGF-2 on survival of HF patients has not been documented in a trial. The experimental studies which evaluated the effect of this somatomedin on inhibition of cardiomyocyte apoptosis indicated an influence similar to IGF-1 (by activating similar metabolic pathways) [29]. Additionally, it emphasizes that IGF-2 has a very strong mitogenic effect on muscle cell growth and differentiation. The IGF-2 mitogenic activity is based on the influence on the cascade mechanism of tyrosine kinase activation signal transduction and it is stronger than IGF-1 [5, 6]. In our study, not only did we find higher IGF-2 levels among patients with less advanced HF, but also the IGF-2 concentration was significantly lower in the patients who died during the 12-month follow-up compared to living patients. We hypothesize that perhaps the “beneficial” IGF-2 effect on cardiomyocytes is more significant than IGF-1. This is also confirmed in the univariate regression model. We determined that lowered IGF-2 concentration is one of the parameters with an established effect on the death risk. We did not observe such an effect for IGF-1.
Previously published studies have shown that the levels of IGFs are influenced by cholesterol concentration [7]. In the present study, we also found that in the NYHA III/IV group patients IGF-2 concentration positively correlated with the total cholesterol and triglyceride concentrations (Figure 2). A similar relationship was also observed in patients with BMI ≥ 25 kg/m2. In this patient group IGF-2 concentration positively correlated with total cholesterol and the LDL fraction (Figure 3). Such correlations were not found for IGF-1.
Our results confirm that increased serum lipids correlate with enhanced IGF-2 secretion. Fatty acids are a proven significant energy source for cardiomyocytes [31, 32].
We can speculate that IGF-2 secretion takes place only if the energy cell reserves are properly “secured”, because lipids are an important energy source and building material for cardiomyocyte growth and differentiation.
In the relation of low values of total and LDL cholesterol, which characterized patients from the NYHA III/IV group [33], the IGF-2 secretion increased only with enhanced concentrations of these lipids. This relationship may explain why in malnourished and cachectic patients with low serum lipids we observe lower values of somatomedins, which may be the reason for development of more advanced HF and higher mortality of such patients [34].
Recently, it was also observed that IGF-2 reduced the concentration of glucose in the plasma more strongly than IGF-1 [35]. Since IGFs are structurally identical to insulin, they can exert the same metabolic effects through specifically located receptors. This includes enhancement of glucose transport to cells, glucose oxidation, and reduction of free fatty acid release. This effect can take place both through the IGF and the insulin receptors [31, 35]. Perhaps, also due to the mechanism of a greater glycemia decrease, IGF-2 also positively influences the prognosis of patients. However, in the present study, we did not find significant differences in IGF-2 levels between patients with and without carbohydrate disturbances.
We speculate that IGF-2 plays a role in the “obesity paradox” in HF, which means a better prognosis for HF patients with excess body weight [36, 37]. The positive correlation of IGF-2 with TC and LDL concentrations that was observed among patients with BMI ≥ 25 kg/m2, often characterized by hyperlipidemia, may be responsible for a stronger effect on the growth and multiplication of muscle cells in this group.
It can be suggested that IGF-2 has a stronger influence on the processes of cellular metabolism (carbohydrates and lipids) than IGF-1, which participates mainly in GH-controlled growth processes. This is a hypothesis that requires confirmation in studies on a larger group of patients and verification in experimental research.
The main limitation is the small size of the study group and the large dispersion of results. The mean IGF-2 concentration in patients from NYHA groups and those who died vs. survived was significantly different, but the SD values of the compared groups overlapped widely. In this situation a single value in an individual patient cannot be considered clinically meaningful or significant. The correlations between IGF-2 and TC and LDL have wide dispersion around the regression line too, only indicating a dependence suggestion. The study results require confirmation in a larger patient group for future research and follow-up studies. Another study limitation concerns the results of echo examination, because intraobserver and interobserver echocardiographic reproducibilities were not calculated.
In conclusion, based on these results it can be inferred that reduced IGF-2 concentration may be a better marker, compared to low IGF-1 concentration, of patients with more clinically advanced HF and a higher death risk in 1-year follow-up. It seems that IGF-2 can stimulate the metabolic processes in heart muscle cells more strongly than IGF-1 and its secretion may be associated with serum lipid levels.