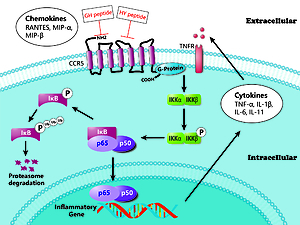
Introduction
Inflammatory bowel disease (IBD) is a group of chronic nonspecific intestinal inflammatory diseases with unknown etiology, including Crohn’s disease, ulcerative colitis and indeterminate colitis [1]. Its prominent pathophysiological features are infiltration of multiple inflammatory cells in the intestine, activation of various inflammatory signaling pathways and the release of multiple inflammatory cytokines, resulting in persistent inflammation and damage to the intestinal mucosa [2].
CC chemokine receptor 5 (CCR5) is a chemokine receptor that belongs to the family of G protein coupled receptors (GPCRs). CCR5 regulates recruitment and activation of several kinds of inflammatory cells [3]. CCR5 is also involved in the pathogenesis of various immune diseases, such as asthma [4], autoimmune myocarditis [5], rheumatoid arthritis [6] and IBD [7]. Structurally, CCR5 contains an extracellular N-terminal, three extracellular loops (ECL1, ECL2 and ECL3), seven transmembrane α-helices, three intracellular loops and an intracellular C-terminal [8]. After binding to its ligands, the intracellular C-terminal, which is coupled with the G protein, is phosphorylated and involved in various signaling pathways, including the MAPK, JAK-STAT and NF-κB signal pathways [9–11].
As a ubiquitously expressed transcription factor, NF-κB has been shown to play a pivotal role in inflammation, immune regulation and cell apoptosis [12]. The NF-κB family is composed of five proteins in mammals: p65/RelA, c-Rel, RelB, NF-κB1 (p105/p50) and NF-κB2 (p100/p52). Among them, p105 and p100 are the precursor proteins of p50 and p52, respectively. In unstimulated conditions, NF-κB is localized to the cytoplasm, where it is tightly bound to the inhibitory protein IκB (inhibitor kappa B) [13]. Several inflammatory stimuli (e.g., TNF-α, LPS, IL-1 and mitogens) can activate the IκB kinase (IKK) complex. Then, the IKK complex phosphorylates IκBs, resulting in the ubiquitination and subsequent degradation of IkBa by the proteasome and the liberation and nuclear translocation of active NF-κB dimers, which are able to bind to DNA and regulate the transcription of multiple genes involved in inflammation and the immune response, such as IL-1β and TNF-α [14].
As one of the key regulators of the immune response, NF-κB is associated with the pathophysiology of IBD. Expression and activation of NF-κB results in the dominance of proinflammatory cytokines such as TNF-α, IL-1β and IFN-γ, which cause colonic mucosal inflammation and damage [15]. On the other hand, many treatments of IBD have been shown to inhibit the NF-κB signaling pathway [16–18]. Therefore, blocking the activation of NF-κB might be an effective target of therapeutic interventions in IBD.
Our previous study showed that CCR5 is closely related to the pathogenesis of IBD and is highly expressed in the colonic mucosa of patients with IBD [19]. Using phage display technology, we successfully screened two bioactive peptides that specifically bind to the first and the second extracellular loops (ECL1 and ECL2) of CCR5 and preliminarily found an inhibitory effect of these peptides on colitis. [20]. In addition, in another related study, we found that the CCR5 antagonist peptides reduce airway inflammation by suppressing the IL-23/Th17 signaling pathway [21]. Therefore, CCR5 is closely related to a variety of autoimmune diseases, including IBD. The purpose of this study was to examine the effects of the two peptides on inflammatory cell infiltration and the NF-κB signaling pathway in the colonic mucosa of TNBS-induced experimental colitis rats.
Material and methods
Reagents
A 5% solution of 2,4,6-trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The rabbit antibodies, including anti-IKKβ, anti-phospho-IKKα/β (Ser176/180), anti-p65, anti-phospho-p65 (Ser536), anti-IκBα and anti-phospho-IκBα (Ser32), were purchased from Cell Signaling Technology (Danvers, MA, USA). The rabbit antibodies, including anti-TNF-α, anti-β-actin, and anti-TBP, were purchased from Novus Biologicals, LLC (Littleton, CO, USA). Two peptides that specifically bind to ECL1 and ECL2 of CCR5 were synthesized by GL Biochem Ltd. (Shanghai, China). The sequences of the two peptides were GHWKVWL (GH) and HYIDFRW (HY). More details of the two peptides including (HPLC and mass spectrum analysis report) are provided in the Supplementary material 1.
Animals and groupings
The study was approved by the institutional animal care and use committee and the experimental animal ethics committee of Sun Yat-sen University. The license number is IACUC-DB-16-0313. Forty female Sprague-Dawley rats weighing 180-220 g were purchased from the experimental animal center of Sun Yat-sen University (Guangzhou, China). After feeding for 1 week, the forty rats were randomly divided into four groups: (1) normal control group (N group), (2) model colitis group (M group); (3) GH peptide treatment group (GH group); and (4) HY peptide treatment group (HY group). The rats were given laboratory chow and tap water in standard cages in a specific pathogen-free (SPF) environment where the temperature was 19–22°C and the humidity was 50–60% with a 12 h day and night cycle. Before induction of experimental colitis, the rats were fasted for 24 h and fed with a 5% glucose solution during that period.
Establishment of experimental colitis
The procedure of inducing colitis was mainly based on Morris et al. [22]. After anesthesia by intraperitoneal injection of 0.3% pentobarbital sodium (0.6 mg/kg), the rats were given an enema with 100 mg/kg TNBS dissolved in 0.3 ml of 50% ethanol via a 16-gauge animal feeding needle (the tip was 8 cm proximal to the anus, approximately at the rat’s splenic flexure). Then, the rats were kept in a head-down position for 5 min to prevent the TNBS from leaking out. None of the rats exhibited signs of peritonitis after the administration of 0.3% pentobarbital sodium.
Drug administration
On the third day after TNBS administration, rats in the treatment groups were treated with GH peptide (35 mg/kg) and HY peptide (25 mg/kg) via tail vein injection, while rats in the normal and model groups were injected with sterile saline (0.9% NaCl). All injections were given once a day and lasted for 7 days. The concentrations of peptides were determined based on our previous study [23], and the results are listed in the supplementary materials (Supplementary material 2). Twelve days after the TNBS enema, the rats were euthanized, and colon tissue samples were collected for further histological and molecular biology analysis. Humane endpoints were evaluated by a clinical scoring system (Supplementary material 3) and rats were anesthetized and euthanized by decapitation when termination criteria were fulfilled or at the end of the experiment.
Histological evaluation
The colon samples were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Then, they were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin. Histological assessments including ulceration, inflammation and types of inflammatory cells were performed under a microscope. Inflammatory cells, including neutrophils, eosinophils, lymphocytes and macrophages, were counted in ten randomly selected high-power fields (HPFs) at 400× magnification.
Protein extraction and western blotting
Total protein lysates were collected by RIPA lysis buffer. The nuclear extracts were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology Ltd; Shanghai, China). Equal amounts of samples (as determined by BCA protein assay) were loaded onto SDS-PAGE gels (20 µl) and transferred to nitrocellulose membranes. After blocking in 5% nonfat milk, the membranes were incubated with the relevant primary antibodies at 4°C overnight. Then, the membranes were probed with HRP-linked secondary antibodies at room temperature for 1 h. Finally, the proteins were visualized by the ECL method (Millipore; Burlington, MA, USA). To control for loading, β-actin and TBP served as the housekeeping proteins for the total and nuclear proteins, respectively.
Real-time PCR
Total RNA was isolated by RNAiso Plus (Takara Bio Inc, Dalian, China). Real-time PCR was performed using SYBR Green PCR master mix reagent kits after reverse transcription to cDNA, and β-actin was used as the housekeeping gene. The real-time PCR cycling parameters were 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. The primer sequences are listed in Table I. All primers were synthesized by Sangon Biotech Ltd. (Shanghai, China).
Table I
Primer sequences for real-time PCR
Statistical analysis
Normality was assessed by the Kolmogorov-Smirnov test. For normal distribution, data are shown as the mean ± standard deviation (SD) and were analyzed by one-way ANOVA and the LSD multiple comparison test. For abnormal distribution, data are shown as the median [interquartile range] and were analyzed by the Kruskal-Wallis H test and Nemenyi multiple comparison test. All statistical analyses were performed using SPSS software (version 22.0). Correlation analysis was assessed by the Spearman correlation test. For all tests, p < 0.05 was considered statistically significant.
Results
Effects of GH and HY peptides on the histological damage and infiltration of inflammatory cells
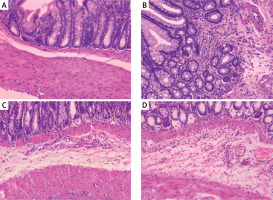
Compared with the histological features in the normal control, the histological features of the colonic mucosa in the model group manifested as mucosal ulceration, epithelial erosion, crypt damage and distortion and infiltration of large numbers of neutrophils and lymphocytes and some macrophages and eosinophils (Figures 1 A, B). However, administration of GH or HY peptide resulted in a decrease in the extent of inflammation (mainly located in the submucosa) (Figures 1 C, D) and infiltration of neutrophils, lymphocytes and macrophages compared with those of the model group. There was no significant difference in the number of inflammatory cells and the morphology of the colonic mucosa between the two peptide groups, and there was no difference in the number of eosinophils in the GH and HY treatment groups compared with that of the model group. The inflammatory cell counts are listed in Table II.
Figure 1
Histological changes in the colonic mucosa in each group (HE × 100). A – Normal control group. The structure of the colonic mucosa was intact. B – Model group. TNBS enema resulted in ulceration, crypt distortion, and severe inflammation throughout the colonic mucosa and infiltration of large amounts of inflammatory cells. C – GH peptide group. D – HY peptide group. Inflammation in the GH and HY groups was mainly located in the submucosa, and infiltration of inflammatory cells was reduced significantly compared with that of the model group
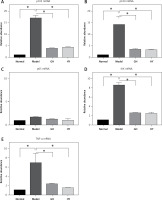
Effects of GH and HY peptides on the gene expression of p100, p105, p65, IKK and TNF-α
The expression levels of NF-κB-related genes, including p105, p100, p65, IKK and TNF-α, were tested by real-time PCR (Figure 2). The mRNA levels of p105, p100, IKK and TNF-α in the model group elevated by approximately 16-, 3.8-, 8- and 6.8-fold, respectively (p < 0.01), compared with those of the normal control group. In contrast, the mRNA levels of p100, p105, IKK and TNF-α in the GH or HY peptide groups were lower than those of the model control group. There was no difference in the expression of p65 mRNA among the four groups (p > 0.05).
Figure 2
Expression of NF-κB-related genes in each group. The results revealed that except for p65, GH and HY peptides significantly inhibited mRNA expression levels of p105 (77% and 74% inhibition for GH and HY peptides, respectively) (A), p100 (74% and 76%) (B), p65 mRNA (C), IKK (70% and 72%) (D), and TNF-α (66% and 78%) (E)(*p < 0.01)
Effects of GH and HY peptides on the protein expression of the NF-κB signaling pathway and the phosphorylation of IKK, IκBα and p65
The expression of NF-κB-related proteins was tested by western blotting. The results showed that the protein levels of p-IKK (phospho-IKK), p-p65 (phospho-p65), p-IκBα (phospho-IκBα) and TNF-α in the model rats were significantly elevated by approximately 1.4-, 1.9-, 1.7- and 1.6-fold, respectively, and the protein level of IκBα was significantly lower than in the normal group (p < 0.05) (Figure 3), indicating a significantly activated NF-κB signaling pathway and degradation of IκBα. In contrast, the GH or HY peptide resulted in decreased levels of p-IKK, p-p65, p-IκBα and TNF-α compared with those of the model group. Compared with the levels in the normal group, there was no difference in the protein levels of IκBα and p-IκBα in the CCR5 peptide treatment groups. Furthermore, no differences were observed in the protein levels of IKK and p65 among the four groups.
Figure 3
GH and HY peptides suppressed the NF-κB signaling pathway and phosphorylation of IKK, IκBα and p65. β-actin was used as a loading control. The results showed that GH or HY peptide reduced the protein levels of p-IKK, p-p65, p-IκBα and TNF-α compared with those of the model control (*P < 0.05)

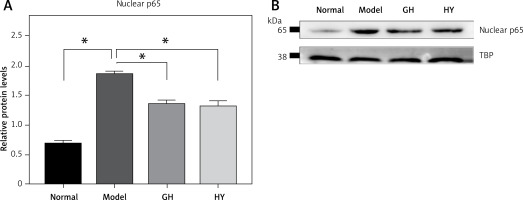
Effect of GH and HY peptides on the nuclear translocation of p65
To further demonstrate whether CCR5 antagonist peptides downregulated NF-κB activity in experimental colitis, we performed western blotting to examine p65 in nuclear extracts. The results showed that in the colitis rats, nuclear p65 was significantly elevated compared with that of the normal control. In contrast, GH or HY peptides reduced p65 in the nucleus compared with that of the model group (p < 0.05). The results are shown in Figure 4.
Figure 4
Effects of GH and HY peptides on the nuclear translocation of p65. Nuclear protein was extracted and analyzed by western blotting for p65 expression. TBP was used as a loading control. Quantification by gray value showed that p65 was reduced by up to 32% and 33% in the GH and HY peptide groups, respectively (*p < 0.05)
Correlation analysis between inflammatory cell infiltration and the NF-κB pathway
Tables III–V show the results of the Spearman correlation analysis of inflammatory cell infiltration and NF-κB pathway expression, when the NF-κB pathway was activated and inhibited, respectively. Parts of the scatter plot results are shown in the supplementary materials (Supplementary Figure S1).
First, a comparison between the normal group and the model group was performed (Table III). The results showed that except for the protein p65, the infiltration of inflammatory cells was significantly correlated with the NF-κB pathway. Among them, the IκBα protein was negatively correlated (ρ = –0.660 to –0.942, p < 0.05), and the others were positively correlated (ρ = 0. 692–0.988, p < 0.05).
Table III
Spearman correlation analysis between N and M groups (when signal pathway was activated)
| Indicators in NF-κB pathway | Neutrophils | Eosinophils | Lymphocytes | Macrophages |
|---|---|---|---|---|
| Proteins | ||||
| IKK | 0.702a | 0.720a | 0.693a | 0.719a |
| pIKK | 0.913a | 0.841a | 0.838a | 0.725a |
| p65 | 0.426 | 0.402 | 0.354 | 0.180 |
| p-p65 | 0.961a | 0.952a | 0.962a | 0.890a |
| IκBα | -0.942a | -0.660a | -0.960a | -0.835a |
| p-IκBα | 0.927a | 0.972a | 0.906a | 0.944a |
| TNF-α | 0.988a | 0.904a | 0.910a | 0.885a |
| nuclear p65 | 0.986a | 0.977a | 0.966a | 0.948a |
| mRNAs | ||||
| p105 | 0.980a | 0.940a | 0.975a | 0.938a |
| p100 | 0.823a | 0.926a | 0.910a | 0.921a |
| IKK | 0.890a | 0.975a | 0.921a | 0.923a |
| p65 | 0.692a | 0.860a | 0.674a | 0.936a |
| TNF-α | 0.864a | 0.920a | 0.890a | 0.929a |
After application of the GH peptide (Table IV), the infiltration of neutrophils, lymphocytes and macrophages was not related to the protein IKK but was related to p-IKK (ρ = 0.918, ρ = 0.821 and ρ = 0.780, respectively, p < 0.05). There was no significant difference in the correlation between the protein p65 and lymphocyte, eosinophil and macrophage infiltration. Except for the above indicators, the infiltration of inflammatory cells was significantly correlated with the NF-κB pathway. Among them, IκBα protein was negatively correlated (ρ = –0.660 to –0.854, p < 0.05), and the others were positively correlated (ρ = 0.590–0.964, p < 0.05).
Table IV
Spearman correlation analysis between N, M and GH groups (when signal pathway was suppressed)
| Indicators in NF-κB pathway | Neutrophils | Eosinophils | Lymphocytes | Macrophages |
|---|---|---|---|---|
| Proteins | ||||
| IKK | 0.323 | 0.774a | 0.260 | 0.455 |
| pIKK | 0.918a | 0.854a | 0.821a | 0.780a |
| p65 | 0.590a | 0.180 | 0.422 | 0.191 |
| p-p65 | 0.826a | 0.938a | 0.878a | 0.964a |
| IκBα | –0.854a | –0.660a | –0.820a | –0.721a |
| p-IκBα | 0.860a | 0.850a | 0.821a | 0.905a |
| TNF-α | 0.945a | 0.880a | 0.908a | 0.877a |
| nuclear p65 | 0.911a | 0.902a | 0.927a | 0.916a |
| mRNAs | ||||
| p105 | 0.926a | 0.689a | 0.962a | 0.826a |
| p100 | 0.839a | 0.663a | 0.904a | 0.865a |
| IKK | 0.933a | 0.695a | 0.903a | 0.870a |
| p65 | 0.658a | 0.930a | 0.699a | 0.844a |
| TNF-α | 0.860a | 0.670a | 0.890a | 0.860a |
After applying the HY peptide (Table V), the infiltration of neutrophils and lymphocytes was not related to the protein IKK but was related to p-IKK (ρ = 0.894 and ρ = 0.831, respectively, p < 0.05). There was no significant difference in the correlation between the protein p65 and neutrophil, lymphocyte and macrophage infiltration. Eosinophil infiltration was not significantly correlated with the proteins p-IκBα and TNF-α and TNF-α mRNA. Except for the above indicators, the infiltration of inflammatory cells was significantly correlated with the NF-κB pathway. Among them, IκBα protein was negatively correlated (ρ = –0.660 to –0.944, p < 0.05), and the others were positively correlated (ρ = 0.496–0.968, p < 0.05).
Table V
Pearson correlation analysis between N, M and HY groups (when signal pathway was suppressed)
| Indicators in NF-κB pathway | Neutrophils | Eosinophils | Lymphocytes | Macrophages |
|---|---|---|---|---|
| Proteins | ||||
| IKK | 0.421 | 0.668a | 0.433 | 0.699a |
| pIKK | 0.894a | 0.502a | 0.831a | 0.706a |
| p65 | 0.149 | 0.560a | 0.091 | 0.124 |
| p-p65 | 0.862a | 0.864a | 0.902a | 0.945a |
| IκBα | –0.944a | –0.660a | –0.892a | –0.761a |
| p-IκBα | 0.894a | 0.352 | 0.882a | 0.862a |
| TNF-α | 0.956a | 0.361 | 0.846a | 0.796a |
| nuclear p65 | 0.905a | 0.830a | 0.927a | 0.929a |
| mRNAs | ||||
| p105 | 0.968a | 0.569a | 0.890a | 0.871a |
| p100 | 0.825a | 0.496a | 0.915a | 0.857a |
| IKK | 0.952a | 0.595a | 0.924a | 0.824a |
| p65 | 0.551a | 0.935a | 0.635a | 0.735a |
| TNF-α | 0.856a | 0.324 | 0.835a | 0.789a |
Discussion
The present study demonstrates that the two CCR5 antagonist peptides can inhibit the infiltration of inflammatory cells and the NF-κB pathway in experimental colitis. Previous studies have shown a close relationship between CCR5 and the pathogenesis of IBD [24–26]. Kucuk et al. [27] found that Met-RANTES, a modified chemokine that internalizes and downregulates CCR5, attenuates colitis and decreases bacterial translocation in experimental colitis. Another study showed that TAK-779, which is a nonpeptide small-molecule antagonist of CCR5, ameliorates dextran sodium sulfate (DSS)-mediated colitis and inhibits the infiltration of monocytes/macrophages and the production of proinflammatory cytokines such as IL-1β and IL-6 [28]. Furthermore, similar results in human IBD demonstrated that CCR5 is highly expressed in the inflamed mucosa and is associated with the disease activity of IBD [19, 29]. These studies established that CCR5 plays an important role in the development of IBD and that CCR5 antagonists might be a novel therapy for IBD.
To date, several CCR5 antagonists have been developed, such as modified chemokines (Met-RANTES) [27], small-molecule agents (TAK-779), monoclonal antibodies (2D7) [30] and short-peptide antagonists. Among them, short-peptide antagonists specifically bind to the extracellular part of CCR5 and have high affinity and safety. In addition, the low cost of production was another advantage of short-peptide antagonists. Therefore, we screened and synthesized two CCR5 antagonist peptides by the phage-displayed peptide library [20] and evaluated the effects of these peptides on TNBS-induced colitis, which is a classic animal model of IBD [31].
Histopathologic assessment revealed that injection of GH or HY peptide reduced the degree and range of inflammation and alleviated the infiltration of neutrophils, lymphocytes and macrophages. Previous studies showed that CCR5 is widely expressed on the cell surface of dendritic cells, monocytes/macrophages, and neutrophils, as well as effector and regulatory T-cells [32, 33]. Our results indicated that since CCR5 is associated with the migration of neutrophils, lymphocytes and macrophages to the inflamed lesion, CCR5 antagonistic peptides might attenuate histological damage and infiltration of neutrophils, lymphocytes and macrophages in experimental colitis by blocking the binding of CCR5 with its ligand. In addition, eosinophils in the model group were higher than those in the normal control group, which was consistent with previous studies [19, 31]. However, the infiltration of eosinophils in the two CCR5 antagonistic peptide-treated groups was not significantly different from that in the model group, which might be due to the low expression of CCR5 on eosinophils.
Previous studies have emphasized the importance of the NF-κB pathway in IBD and TNBS-induced colitis [34–36]. Our study found that the expression of p105, p100, IKK and TNF-α increased in TNBS-induced colitis but decreased after treatment with GH or HY peptides, indicating that GH and HY peptides inhibit the transcription potential of NF-κB-related genes. Western blot results showed that the expression levels of p-IKK, p-p65, p-IκBα and TNF-α increased in the model rats compared with those of the normal control. After treatment with GH or HY peptide, these increased proteins were reduced significantly. Since IκBα is a key molecular target involved in the regulation of the NF-κB signaling pathway, we examined the protein levels of IκBα in the four groups and observed high levels of IκBα in the normal control group, while in the colitis model, the level of degradation of IκBα was up to approximately 42% (by measuring the gray value) (Figure 3). In contrast, the levels of IκBα increased further in rats administered GH or HY peptide. Because the application of GH and HY peptides also suppressed the phosphorylation of IKK, we suggest that the inhibitory action of GH and HY peptides on the NF-κB signaling pathway lies upstream of the activation of the IKK complex and phosphorylation of IκBα and p65. Moreover, TNF-α, which is an important product and inducer of NF-κB, was also inhibited by the CCR5 antagonist peptides. These results were consistent with previous PCR data on the ability of GH and HY peptides to inhibit NF-κB-related genes.
To further verify the inhibition of NF-κB activity by GH and HY peptides, we examined the level of p65 in nuclear extracts. The reduction in p65 in the nucleus indicated that application of GH and HY peptides suppressed the nuclear translocation of p65, rather than changing the total amount of p65. This result could also explain why no differences were found in the mRNA and total protein levels of p65 among the four groups. In summary, after binding to CCR5, the two CCR5 antagonist peptides inhibit the function of CCR5 and activation of the IKK complex, reducing the phosphorylation of IκB, stabilizing p65, and ultimately suppressing the nuclear translocation of p65.
To investigate the relationship between inflammatory cell infiltration and the NF-κB pathway, we analyzed the relationship between inflammatory cell counts and western blot and PCR results. When the NF-κB pathway was activated, except for protein p65, the infiltration of inflammatory cells was correlated with the other indicators in the NF-κB pathway. This result suggests that the infiltration of inflammatory cells is closely related to the activation of the NF-κB pathway, which was also consistent with other studies [37]. In addition, correlation analysis showed that the infiltration of eosinophils was also related to the NF-κB pathway, but the results of inflammatory cell counts under the microscope showed that there was no significant difference in the degree of eosinophil infiltration between the treatment and model group. This may be due to the small number of eosinophils in each group or the low expression of CCR5 in eosinophils [19].
Studies have shown that CCR5 is essential for activation of the NF-κB pathway during viral infection [38]. In this study, we also explored the effects of the two CCR5 antagonist peptides on the NF-κB pathway. The correlation results showed that the two peptides had different effects on inflammatory cell infiltration and the NF-κB pathway, but there was no significant difference between the two peptides in the previous PCR and WB analysis. This may provide ideas for further study of CCR5 antagonist peptides: the ECL1-binding peptide may focus on inhibiting the phosphorylation of IKK in neutrophils and lymphocytes, whereas the ECL2-binding peptide may affect the phosphorylation of IKK in neutrophils. In general, inflammatory cell infiltration was associated with most NF-κB pathway markers after intervention with the CCR5 antagonist peptides. This suggests that CCR5 antagonist peptides are likely to inhibit inflammatory cell infiltration by inhibiting the expression and activation of the NF-κB pathway, thereby reducing histological damage due to colitis in rats. The overall effect of CCR5 binding peptides on the NF-κB pathway is demonstrated in Figure 5.
It was noteworthy that there were no significant differences in histology or protein or gene levels between the GH group and HY group. Different studies on CCR5 functional binding sites have reached various conclusions. Early studies on CCR5 and HIV-1 in CD4+ T cells suggested that the N-terminus and the second ECL of CCR5 played critical roles in HIV-1 coreceptor function [39]. In 2013, Schnur et al. [40] mapped and examined the interactions and affinity of the extracellular domain of CCR5 with its chemokine RANTES by nuclear magnetic resonance (NMR). In terms of the single structure, the N-terminal and ECL2 were found to have higher affinities in binding to RANTES. However, the binding affinity of ECL1-ECL2 with RANTES was obviously stronger than that of ECL2 alone. This was due to the conformational stabilization of ECL2 induced by ECL1. In other words, blocking either ECL1 or ECL2 alone would lead to a reduction in the binding affinity of CCR5 to its ligands. Therefore, blocking both ECL1 and ECL2 at the same time may more strongly inhibit CCR5.
In conclusion, our study indicates that CCR5 antagonist peptides that specifically bind to ECL1 and ECL2 of CCR5 inhibit the infiltration of neutrophils, lymphocytes and macrophages in the colonic mucosa of rats with colitis by inhibiting the activation of the NF-κB pathway. Thus, the application of CCR5 antagonist peptides might be a potential therapeutic approach for treating IBD. Further research on the pharmacokinetics and the synergistic effects of the two peptides will be beneficial for future clinical applications.