Introduction
Radiation therapy is the mainstay of definitive therapy for non-metastatic nasopharyngeal carcinoma (NPC). However, it only successfully controls 50–70% of advanced tumors, and local recurrence and distant metastasis are the major causes of treatment failure. Multiple studies have reported the use of chemotherapy in combination with radiotherapy for the management of locoregionally advanced NPC [1], as NPC is sensitive to chemotherapy.
Targeted therapy has been the main focus of cancer research in the past decade, and more than 10 targeted therapeutic agents are approved by the Food and Drug Administration annually. Molecular targeted therapies, including epidermal growth factor receptor (EGFR) monoclonal antibodies, EGFR tyrosine kinase inhibitors, and vascular endothelial growth factor receptor inhibitors, have shown potential for treating head and neck cancer [2].
Epidermal growth factor receptor is a receptor tyrosine kinase of the ErbB family that plays a critical role in cellular proliferation, differentiation, and survival. Epidermal growth factor receptor is commonly overexpressed or abnormally activated in squamous cell carcinoma of the head and neck, and it is an ideal target for anti-cancer therapy. Epidermal growth factor receptor overexpression is found in more than 80% of patients with locoregionally advanced NPC, and it is associated with shorter survival. Therefore, administration of an anti-EGFR monoclonal antibody is a feasible strategy in locoregionally advanced NPC [3]. However, anti-EGFR molecular-targeted therapies in the clinical treatment of NPC are not an adequate alternative to traditional platinum-based chemotherapy, and their role is only to strengthen the synergy of radiotherapy and chemotherapy. Therefore, they are used mainly in combination with radiotherapy or chemoradiotherapy. Nimotuzumab (Nimo, h-R3), a humanized monoclonal antibody against EGFR, has significant anti-tumor, pro-apoptotic, anti-angiogenic, and radiosensitizing activities. There are emerging data demonstrating the benefits of Nimo in different cancer types [4, 5].
Little is known about the application of Nimo in NPC during a long course of chemoradiotherapy. Moreover, the appropriate timing and best sequential mode to achieve the greatest clinical benefit of combination therapy are unknown. Some retrospective studies have reported that Nimo combined with concurrent chemoradiotherapy (CCRT) shows encouraging outcomes in the treatment of locally advanced NPC, with no increased toxicity and an improved tolerance [6–8]. However, it is unclear whether Nimo combined with induction chemotherapy (IC) or CCRT is the best option. To answer this question, we designed a prospective study to investigate the best mode of Nimo in combination with chemoradiotherapy in the treatment of NPC.
Material and methods
Study subjects
Patients diagnosed with NPC between September 2009 and February 2014 at our institution were considered for enrollment. Inclusion criteria were as follows: 17–73 years old; objective measurement or evaluation of the lesions; Karnofsky Performance Status Scale ≥ 70; blood evaluations: white blood cell (WBC) count ≥ 4.0 × 109/l, platelet count ≥ 100 × 109/l, hemoglobin ≥ 100 g/l; total bilirubin ≤ 1.5 × the upper-normal limit (UNL), aspartate aminotransferase/alanine aminotransferase ≤ 1.5 × UNL, and serum creatinine ≤ 1.5 × UNL; no known allergies; no history of mental illness; and no drug abuse or other unhealthy habits. Patients with metastasis or malignancy in other organs were excluded. Patients were also excluded from the study if they had a history of another malignant tumor, participated in other clinical trials, had severe allergies, were pregnant or lactating, had previously received anti-EGFR therapy, or exhibited no tolerance to therapy.
The initial examination included a detailed medical history, assessment of performance status, physical examination, chest radiography or computed tomography (CT) scan, abdominal B ultrasonic scan or CT scan, bone emission CT scan, blood biochemical analysis, and intensive magnetic resonance imaging (MRI) or CT scan of the nasopharynx and neck. Cases were required to be staged according to the American Joint Committee on Cancer, 2010 Edition (7th, AJCC 2010).
The study protocol was approved by the Research Ethics Committee of the Eye, Ear, Nose & Throat Hospital of Fudan University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Study design and case grouping
This was a prospective, non-randomized trial, and the baseline demographic and clinical pathological characteristics and radiotherapy techniques were well matched. One hundred eighty patients diagnosed with NPC from September 2009 to February 2014 were enrolled, and they were divided into two groups with a sample ratio of 1 : 2 based on administration of Nimo. All the patients in our cohort completed 2–3 cycles of IC followed by CCRT, and 60 of the 180 patients were additionally administered Nimo. According to the application time of Nimo, the patients were divided into three groups: group A (120 patients), IC followed by CCRT; group B (30 patients), in which Nimo was applied at the beginning of IC (IC (+ Nimo) + CCRT); and group C (30 patients), in which Nimo was applied at the beginning of radiotherapy (IC + CCRT (+ Nimo)).
The primary study outcomes were overall survival (OS) and progression-free survival (PFS). The secondary outcome was the development of common toxicities, including hematological toxicity, skin reaction, mucositis, nausea/vomiting, and hepatotoxicity. The OS was defined as the time from the date of diagnosis to the date of death from any cause; PFS was defined as the time between the date of diagnosis and the date of local failure or distant metastasis. The investigators who assessed the treatment outcomes were blinded to the patients’ group assignments.
Treatments
All patients received definitive intensity modulation radiation therapy (IMRT) or three-dimensional conformal radiotherapy (3D-CRT). Prescribed doses were determined according to the tumor volume and stage in reference to IMRT target dose designs and the expert consensus for NPC by the Chinese clinical NPC working committee and Wang’s target delineation protocol [9]. The gross tumor volume doses were delivered with 66–69.75 Gy for the IMRT group and 68–73 Gy for the CRT group, and the treatment dose to involved lymph nodes was 60–70 Gy. The clinical tumor volume dose was delivered with 50–62 Gy.
IC regimens included TPF (docetaxel + cisplatin + 5-fluorouracil (5-FU)), TP (docetaxel + cisplatin), or PF (cisplatin + 5-FU). The TPF protocol consisted of 2–3 cycles of docetaxel 70 mg/m2 on day 1 over 1 h, cisplatin 75 mg/m2 on day 1 to day 3, and 5-FU 500 mg/m2 from day 1 to day 4 as an intravenous infusion every 3 weeks. The PF protocol was the same as the TPF protocol except for the inclusion of docetaxel. Concurrent chemotherapy treatment regimens consisted of 1–3 cycles of cisplatin 80 mg/m2 divided evenly across 3 days, and was repeated every 3 weeks. Dose modifications were allowed during chemotherapy according to the patient’s tolerance, and the chemotherapy application time was adjusted appropriately. Chemotherapy was delayed until observation of a WBC count ≥ 3.5 × 109/l and an absolute neutrophil count ≥ 1.5 × 109/l.
Nimo was administered weekly at a dose of 200 mg in 250 ml of physiological saline. Intravenous infusion of Nimo was administered over 60 min. Dexamethasone 5 mg was administered intravenously before Nimo to prevent allergic reactions. After infusion of Nimo, the infusion apparatus was flushed with normal saline to avoid mixed infusion with other drugs. Six to 8 weeks of the targeted therapy was considered a complete dosage schedule.
Follow-up
Post-treatment assessment of patients, including a physical examination, was scheduled every 8 weeks in the first year, every 12 weeks in the second year, and every 4–6 months thereafter. MRI/CT scan of the nasopharynx and neck, chest radiography, and abdominal ultrasound were performed approximately every 6 months during follow-up. An MRI/CT scan was performed for patients with clinical suspicion of local or locoregional recurrence. Distant metastases were diagnosed by clinical symptoms, physical examinations, chest CT, bone scan, MRI, CT, abdominal CT, or positron emission tomography/CT. The last recorded follow-up was August 25, 2017.
Statistical analysis
Numerical variables are expressed as the mean ± standard deviation (M ± SD), and categorical variables are expressed as a percentage (%). Categorized variables were compared using the χ2 test. Kaplan-Meier curves were used to characterize OS and PFS, and differences in survival between patient subsets were evaluated using the log-rank test. A two-tailed p-value of less than 0.05 was considered statistically significant. All statistical analysis and graphing were performed using IBM SPSS 20 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 168 patients with complete clinical and follow-up data were included in the analysis, including 126 men and 42 women, and 89.9% (151/168) of patients were diagnosed with advanced stage disease. The median age at diagnosis was 49 years (range: 17–72 years). Clinical data are shown in Table I. All the patients received 2–3 cycles of IC, and 20.8% (35/168) of patients underwent dose or time modification of chemotherapy during the course of CCRT. Fifty-six patients received 6–8 cycles of Nimo, and no dose or time modification occurred.
Table I
Baseline characteristics of patients
| Variable | Group A, n (%) | Group B, n (%) | Group C, n (%) | P-value* |
|---|---|---|---|---|
| Sex: | 0.558 | |||
| Male | 84 (75.0) | 20 (69.0) | 22 (81.5) | |
| Female | 28 (25.0) | 9 (31.0) | 5 (18.5) | |
| Age [years]: | 0.501 | |||
| > 50 | 63 (56.3) | 14 (48.3) | 18 (66.7) | |
| ≤ 50 | 49 (43.7) | 15 (51.7) | 9 (33.3) | |
| T classification: | 0.791 | |||
| T1–2 | 62 (55.4) | 16 (55.2) | 13 (48.1) | |
| T3–4 | 50 (44.6) | 13 (44.8) | 14 (51.9) | |
| N classification: | 0.655 | |||
| N0–1 | 26 (23.2) | 9 (31.0) | 6 (22.2) | |
| N2–3 | 86 (76.8) | 20 (69.0) | 21 (77.8) | |
| Clinical stage: | 0.362 | |||
| II | 11 (9.8) | 2 (6.9) | 4 (14.8) | |
| III | 68 (60.7) | 14 (48.3) | 12 (44.4) | |
| IVA–B | 33 (29.5) | 13 (44.8) | 11 (40.8) |
Efficacy
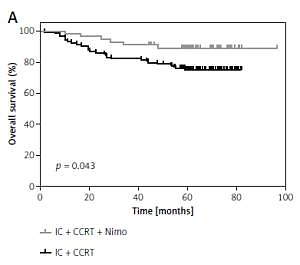
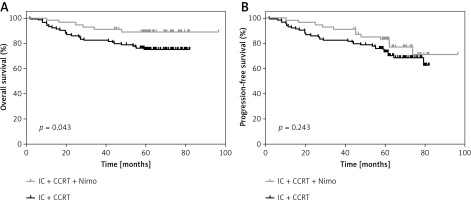
Through August 25, 2017, the median follow-up time was 61.4 months (range: 1.7–96.5 months). The 5-year OS for patients who did not receive Nimo (group A) and those who did (groups B + C) was 74.8 ±4.1% and 87.0 ±4.6%, respectively (p = 0.043; Figure 1 A). During follow-up, 34 patients who did not receive Nimo and 13 patients who did receive Nimo experienced disease progression, and the median times to disease progression were 26.6 and 45.3 months, respectively. The 5-year PFS for patients who did not receive Nimo and those who did was 72.7 ±4.3% and 83.1 ±5.1%, respectively (p = 0.243) (Figure 1 B).
Figure 1
Kaplan-Meier estimation of 5-year-OS (A), 5-year-PFS (B) for patients who received IC plus CCRT treatment regimen with Nimo (n = 56) or without Nimo (n = 112)
P-values were calculated with log-rank test. IC – induction chemotherapy, CCRT – concurrent chemoradiotherapy, Nimo – nimotuzumab.
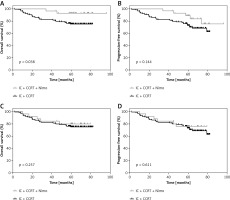
There was a striking difference in the 5-year OS rate between group B and group A (93.0 ±4.8% vs. 74.8 ±4.1%, p = 0.038; Figure 2 A). However, no significant difference in 5-year PFS was found between group B and group A (89.3 ±5.9% vs. 72.7 ±4.3%, p = 0.144, Figure 2 B). The 5-year OS and PFS for group C were 80.4 ±7.9% and 76.4 ±8.5%, respectively, and there was no significant difference compared with those of group A (p = 0.257 and p = 0.611, respectively; Figures 2 C and D).
Figure 2
Kaplan-Meier estimation of 5-year-OS (A), 5-year-PFS (B) for patients who received IC concurrent Nimo plus CCRT (n = 56) or IC plus CCRT (n = 112), 5-year-OS (C), 5-year-PFS (D) for patients who received IC plus CCRT concurrent Nimo (n = 56) or IC plus CCRT (n = 112)
P-values were calculated with log-rank test. IC – induction chemotherapy, CCRT – concurrent chemoradiotherapy, Nimo – nimotuzumab.
Adverse events
Graded adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. The treatment toxicities were generally mild, and no grade 3–4 acute toxicities occurred in the IC setting in any of the patients. In the concurrent chemotherapy phrase, nausea and vomiting were common in patients who received cisplatin. The other common acute adverse effects were oral mucositis, leukocytopenia, and skin reaction; however, there was no significant difference in toxicities among the different treatment regimens. It was rare to find a specific toxicity that was induced by Nimo. The majority of acute toxicities rapidly disappeared, and most patients recovered in a few months. Detailed adverse effects are displayed in Table II.
Table II
Acute toxicities in different treatment regimens
| Adverse events | Group A, n (%) | Group B, n (%) | Group C, n (%) | P-value* |
|---|---|---|---|---|
| Anemia: | 0.965 | |||
| G1–2 | 26 (23.2) | 7 (24.1) | 6 (22.2) | |
| G3–4 | 5 (4.5) | 1 (3.4) | 1 (3.7) | |
| Thrombocytopenia: | 0.964 | |||
| G1–2 | 22 (19.6) | 6 (20.7) | 5 (18.5) | |
| G3–4 | 5 (4.5) | 1 (3.4) | 1 (3.7) | |
| Leukocytopenia: | 0.987 | |||
| G1–2 | 50 (44.6) | 13 (44.8) | 13 (48.1) | |
| G3–4 | 4 (3.7) | 1 (3.4) | 2 (7.4) | |
| Skin reaction: | 0.87 | |||
| G1–2 | 91 (81.3) | 24 (82.8) | 23 (85.2) | |
| G3–4 | 6 (5.4) | 1 (3.4) | 1 (3.7) | |
| Nausea/vomiting | 63 (56.3) | 16 (55.1) | 15 (55.6) | 0.994 |
| Hepatoxicity | 17 (15.2) | 3 (10.3) | 3 (11.1) | 0.727 |
| Mucositis: | 0.977 | |||
| G1–2 | 103 (92.0) | 27 (93.1) | 25 (92.6) | |
| G3–4 | 9 (8.0) | 2 (6.9) | 2 (7.4) |
Xerostomia was the most common late adverse effect, and the degree of xerostomia appeared to decrease with time. Some patients complained of hearing impairment and recurrent secretory otitis media, and were treated in the Department of Otorhinolaryngology.
Discussion
This is a prospective, non-randomized trial of Nimo combined with IC followed by chemoradiotherapy for advanced NPC.
At present, IC + CCRT, CCRT, and CCRT plus adjuvant chemotherapy are the three major sequential modalities used to treat advanced NPC [10]. Studies have shown that CCRT plus adjuvant chemotherapy provides significant survival benefits over RT alone; however, it is also accompanied by intolerable toxicities [11–14]. According to the 2013 National Comprehensive Cancer Network guidelines for head and neck cancer, CCRT is the standard treatment for patients with advanced NPC. However, increasing clinical trials have shown the feasibility and efficacy of IC for the treatment of advanced NPC. IC has the potential advantage of shrinking the tumor bulk before irradiation and, as a result, narrowing the radiation dose distribution difference caused by change in tumor size. Addition of TPF IC to CCRT significantly improves failure-free survival in locoregionally advanced NPC with acceptable toxicity [15].
EGFR has been evaluated as a therapeutic target for NPC, as there is increasing evidence that the EGFR signaling pathway might be important in NPC pathogenesis [16]. Anti-EGFR monoclonal antibodies have improved survival in patients with advanced head and neck cancer [17, 18]. Ma et al. [19] suggested that the use of cisplatin combined with cetuximab to treat locally advanced NPC was feasible. Nimo, a human EGFR monoclonal antibody, can antagonize epidermal growth factor and growth factor-α to block the EGFR extracellular domain structure, inhibiting tyrosine kinase activity and cell proliferation, thereby exhibiting anti-tumor capability [20]. Interestingly, however, Westphal et al. [21] found that there was no obvious correlation of Nimo efficacy with EGFR status. Nimo was proven to increase the cytotoxic effect of radiation and chemotherapy on tumor cells in advanced head and neck tumors [22]. Two retrospective analyses with a large cohort of patients with stage II–IV b NPC suggested that Nimo administered concurrently with IMRT might improve the survival benefit in patients with advanced NPC over CCRT alone [6, 23]. Li et al. [24] believed that Nimo + RT should only be administered to patients with stage II NPC, those older than 60 years, and those resistant to cisplatin.
Nimo was safe and well tolerated with few mild to moderate self-limiting adverse events. There are few studies on the best combination of Nimo and chemoradiotherapy to achieve the maximum therapeutic effect. In this study, we investigated the difference among 3 sequential modalities of Nimo in combination with chemoradiotherapy. We found that Nimo plus IC followed by CCRT achieved the best survival benefit for advanced NPC.
Regardless of when Nimo was administered, we observed a significant improvement in 5-year OS (87.0 ±4.6% vs. 74.8 ±4.1%, p = 0.043) and a non-significant improvement in PFS (83.1 ±5.1% vs. 72.7 ±4.3%, p = 0.243) in patients who received Nimo. Therefore, we conclude that Nimo combined with chemoradiotherapy is effective for advanced NPC. In further analysis, we found that when Nimo was applied simultaneously with IC, the 5-year-OS was significantly higher than that in patients who did not receive Nimo (p = 0.038); however, there was no difference in 5-year OS or PFS between patients who received Nimo at the beginning of CCRT and those who did not receive Nimo. Therefore, Nimo concurrent with IC followed by CCRT might be the optimal mode of sequential treatment for patients with advanced NPC. The results in our study were quite different from the current thinking in the field, which could be because all the published data are based on the application of Nimo with CCRT [25–27]. To the best of our knowledge, there are few studies reporting that Nimo can be applied concurrently with IC to treat NPC. Moreover, this treatment mode achieved better survival benefits. Nimo might assist the IC to control the primary tumor and the locoregional lymph nodes. In addition, the weekly application of Nimo could also contribute to patient well-being, because many patients did not believe they were treated promptly during the IC interval.
Compared with cetuximab, another EGFR inhibitor, Nimo showed a great advantage in terms of toxicity and tolerance. In our study, the proportion of common adverse events did not increase with the addition of Nimo to chemoradiotherapy. In addition, our results demonstrated that no Nimo-related skin rash was found in any patient. Nimo was well tolerated, especially when combined with IC. The results of this study were similar to those of previous studies [8]. However, in our experience, the tolerance and compliance of patients who received Nimo with IC were better than those of patients who received Nimo combined with CCRT. The former were more likely to adhere to the treatment without delay or dose modification.
However, due to the small number of patients administered Nimo and the non-randomized design of this trial, further randomized controlled phase III clinical research is required. A retrospective study of large samples similar to this study is ongoing, and we expect a promising result soon.
In conclusion, based on our present data, the addition of Nimo to chemoradiotherapy was more effective in improving the overall survival rate of patients with nasopharyngeal carcinoma compared to chemoradiotherapy alone. Nimotuzumab administered with chemoradiotherapy was effective for advanced nasopharyngeal carcinoma. However, Nimo concurrent with induction chemotherapy followed by concurrent chemoradiotherapy could be the optimal mode of sequential treatment for this combination.