Introduction
The genus of Piper is distributed in the tropical region, especially in Southeast Asia countries including Malaysia, Thailand and Indonesia. Piper sarmentosum, which belongs to the Piperaceae family, locally known as ‘kaduk’, is a herbal plant that has a wide usage in traditional medicine. It is a glabrous, creeping terrestrial herbaceous plant with aromatic odour and pungent taste [1], which grows as a stoloniferous shrub, to about 0.3–0.8 m in height. The leaves are ovate shape, 5–10 cm wide and 7–15 cm long. Most parts of the plant have potential benefits. In Malaysia, Piper sarmentosum leaves and root are applied to the forehead to relieve headache, while its decoction is known to relieve muscle weakness and pain [2]. In Thailand, the roots are used for stomach ache, while the leaves have been shown to reduce gastritis [3].
Studies have shown that diverse causes of gastritis are non-steroidal anti-inflammatory drugs, alcohol and stress. Despite the different causes, a common factor implicated at the molecular level in the pathogenesis of this clinical entity is free radicals [4, 5], which overwhelm the endogenous antioxidant system. Oxygen-derived free radicals are cytotoxic and mediate tissue damage by injuring cellular membranes and releasing intracellular components. It has been shown that agents with the ability to catalytically reduce free radicals or act as antioxidants can protect the gastric mucosa against a variety of noxious stimuli [6, 7].
Responses to stress exposure are important for human survival. Although this is true, repeated activation of stress responses as well as sustained activation have been shown to cause overexposure to stress hormones, thereby increasing the risk of various health problems [8]. One common health problem related to stress is the formation of gastric lesions, better known as stress ulcers. A variety of stress models have been used in a laboratory setting. One of the most reproducible results can be obtained by a water-immersion restraint stress model. This is due to the fact that immobilisation accompanied by immersion in water acts synergistically to produce gastric lesions. This model had been used in many studies to mimic stress ulcers in critically ill patients due to its reproducibility [9], where critically ill patients are prone to various levels of stress.
Methanolic extracts of Piper sarmentosum leaves were found to have a higher level of antioxidant activity compared to other traditional medicine plants [10]. They were found to possess high antioxidant activity, which might be attributed to the chemical components present in the plant such as vitamins C and E, xanthophylls, carotenes and phenols [10]. There are still minimal numbers of scientific studies which report on the effects of Piper sarmentosum, although it is widely used as an alternative medicine, and there is relatively little information pertaining to its possible use to inhibit oxidative stress in gastric tissues. The effects of Piper sarmentosum on oxidative stress could account for the beneficial effect of this herb in a model of stress-induced gastric injury. Since the leaves are used in traditional folk medicine, it is therefore of interest to investigate the ability of Piper sarmentosum to reduce gastric injuries and to compare its effect with omeprazole, a well-known pharmacological agent used for treatment of gastric ulcers, assessing parameters involved in stress-induced gastric lesions.
Material and methods
Plant materials
Fresh leaves of Piper sarmentosum were collected from the Forest Research Institute Malaysia (FRIM) reserve forest and were authentically identified by the Medicinal Plant Division, FRIM. A voucher specimen (FRI 45870) was deposited at the Medicinal Plant Division, FRIM.
Preparation of methanolic extract of Piper sarmentosum
Fresh leaves of the plants were cleaned with tap water and dried at room temperature before being chopped into small pieces. The extraction procedure followed Sawangjaroen et al. [11]. In brief, 250 g leaves were mixed with 2.5 l of methanol. This mixture was heated using Soxhlet at 45–60°C, after which the methanol undergoes evaporation. The paste material produced was kept at 4°C until use. The percentage yield from the crude dried extract is ≈ 10%.
Experimental animals
Male Wistar rats (n = 28) were obtained from the Animal Unit, Faculty of Medicine, Universiti Kebangsaan Malaysia. All rats were kept on a regular night/day cycle, with natural light for a period of 10 h (07:00 to 17:00 h). Throughout the feeding period all rats were habituated to handling to reduce their stress-related disturbances. The rats were housed in large cages with wide wire-mesh bottoms to prevent coprophagy. Food and water were given ad libitum throughout the experiment. Prior ethical approval was obtained from Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC).
Design of treatment
The rats were divided into four equally sized groups. Two control groups were fed with normal rat diet (NS and S), while the treatment groups received the same diet but with oral supplement of Piper sarmentosum (PS) or omeprazole (OMZ) at 500 mg/kg or 20 mg/kg body weight respectively for 28 days. Piper sarmentosum and omeprazole were dissolved in vitamin-free palm oil as a vehicle and were administered by oral gavage using an 18G gavage needle. The control groups were sham-administered with vitamin-free palm oil. At the end of the treatment period, the rats from one control group (stressed control) and both of the treated groups were exposed to water-immersion restraint stress (WIRS). After the last exposure to stress the rats were sacrificed. The dissected stomach was taken for evaluation of gastric lesions, gastric acidity, malondialdehyde and prostaglandin E2 levels. The measurement was done after the rats were sacrificed by overdose of anaesthesia.
Water-immersion restraint stress procedure
Rats were restrained by placing them in individual plastic restrainer measuring approximately 17 × 5 cm and immersing them in water neck deep for 3.5 h once, as previously described by Ibrahim et al. [12]. Following the stress procedure the rats were sacrificed, and the stomach was dissected along the greater curvature and examined for lesions.
Macroscopic assessment of stress-induced gastric lesions
The macroscopic assessment of stress-induced gastric lesions in the gastric mucosa was performed by two independent examiners who were blinded to the treatment that the rats received. The assessments of lesions were done according to a semi-quantitative score. The score used was as follows: 5 = generalised haemorrhage or haemorrhagic lesions covering more than 90% of the gastric mucosa; 4 = haemorrhage covering 60–89% of the gastric mucosa; 3 = haemorrhage covering 30–59% of the gastric mucosa; 2 = haemorrhage covering 10–29% of the gastric mucosa; 1 = generalised erythema with presence of haemorrhage; and 0 = no visible lesion.
Determination of gastric acidity
Measurement of the gastric acidity was done following a method described by Shay et al. [13]. The junctions between the stomach-oesophagus and duodenum-pylorus were secured before the stomach was isolated. Then, 3 ml of distilled water was introduced into the stomach and the organ was carefully shaken. The gastric juice was then collected and centrifuged for 10 min at 3000 rpm. The supernatant was then taken and diluted 10 times. Following this, a few drops of phenolphthalein were added to the solution. Titration was done using 0.01 M solutions until the colour of the test solution changed to light pink indicating pH 7.0. The volume of sodium hydroxide (NaOH) needed for titration was used in the calculation to derive the hydrogen ion concentration.
Measurement of gastric malondialdehyde content
The content of malondialdehyde (MDA) in the stomach was determined using the method described by Ledwozyw et al. [14]. A sample of 0.5 ml was acidified with 2.5 ml of 1.22 mol/l trichloroacetic acid in 0.6 mol/l HCl. The mixture was left to stand for 15 min. After this time, 1.5 ml of 0.6% thiobarbituric acid in 0.05 mol NaOH was added. The sample was then incubated in a 100°C water bath for 30 min. Subsequently it was cooled under running tap water and 4 ml of n-butanol was added. After thorough mixing, the mixture was centrifuged for 10 min at 1500 xg. The absorbance of the upper layer was read at 535 nm.
Measurement of gastric prostaglandin E2 content
Sample preparation for prostaglandin E2 (PGE2) assay was done using the method previously described by Redfern et al. (1987). Gastric PGE2 content was measured using an EIA kit (514010, Cayman, USA).
Cyclooxygenase mRNA quantitation
For COX-1, COX-2 and GAPDH mRNA quantitation, the standard QuantiGene Plex 2.0 assay kit (Genospectra, Fremont, CA) protocol was followed. Briefly, the tissue lysate was transferred to a capture well in the presence of the gene-specific probe set and then hybridised at 53°C overnight. Wells were washed twice with bDNA wash buffer and then incubated at 46°C sequentially with an amplifier and an alkaline phosphatase-linked label probe with a wash step between the incubations. After the final wash step, addition of streptavidin phycoerythrin (SAPE) generated a signal that was proportional to the amount of target RNA present in the sample. The luminescence signal was detected using a Luminex instrument. The protocol followed was as previously described by Zhang et al. [15].
Statistical analysis
All data were tested for normal distribution and presented as mean ± standard error (SEM). Data were analysed by a one-way analysis of variance (ANOVA) followed by Tukey test for multiple group comparisons. A value of p < 0.05 was taken as significant. All statistical analysis was conducted using the SPSS version 20 software.
Results
Piper sarmentosum effect on stress-induced gastric lesions
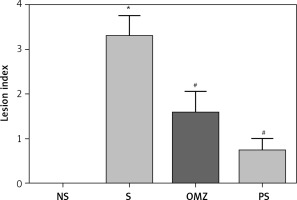
Rats exposed to water-immersion restraint stress for 3.5 h showed presence of considerable ulcerogenicity in the form of gastric erosion and haemorrhagic mucosal lesions. As shown in Figure 1, the gastric lesions index in the stressed control (S) group was 74% higher compared to the PS group and 48% compared to the OMZ group. These findings indicate that both Piper sarmentosum and omeprazole are able to reduce the formation of stress-induced gastric lesions. Rats killed after a 28-day feeding period and not exposed to stress had no gastric mucosal lesions.
Piper sarmentosum effect on gastric acidity in stress
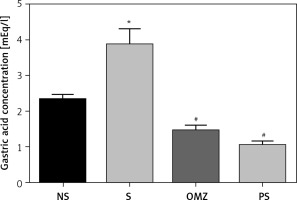
The mean gastric acid concentration in rats exposed to restraint stress was significantly higher (p < 0.05) compared to the non-stressed controls, as shown in Figure 2. The findings suggest that stress does alter the gastric acid concentration. Supplementation with either OMZ or PS reduced gastric acid concentration towards the non-stressed control values.
Piper sarmentosum effect on gastric malondialdehyde in stress
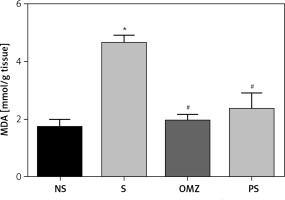
As shown in Figure 3, treatment of rats with both PS (p = 0.046) and OMZ (p = 0.0042) individually causes a significant reduction in gastric MDA level compared to stressed controls. Stress causes an increase in gastric thiobarbituric reactive substance (TBARS) as indicated by the increase of gastric MDA content.
Piper sarmentosum effect on gastric prostaglandin E2 in stress
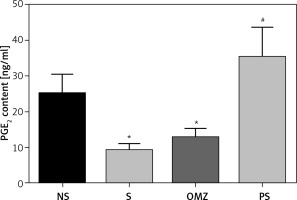
Exposure to water-immersion restraint stress also resulted in a significant reduction of gastric PGE2 content by 21.2% (p = 0.001) in the stressed control compared to the non-stressed control (NSC) group, as shown in Figure 4. Rats treated with PS showed an increase in PGE2 content in stressed controls. However, supplementation with OMZ did not have the effect of maintaining PGE2 content in the non-stressed levels.
Piper sarmentosum effect on gastric cyclooxygenase enzymes in stress
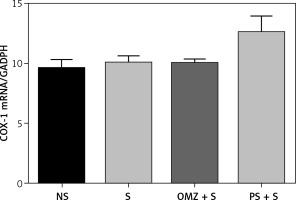
The ratio of COX-1 mRNA and GAPDH in the intact mucosa was comparable to that of the rats that were exposed to stress and developed gastric lesions and rats treated with omeprazole, as shown in Figure 5. However, the COX-1 and GAPDH ratio was higher in the PS treated group, although the difference was not significant.
Figure 5
Effects of Piper sarmentosum (500 mg/kg) (PS) and omeprazole (20 mg/kg) (OMZ) on gastric COX-1 mRNA expression in rats exposed to water immersion restraint stress (n = 7)
*vs. non-stressed control (NS) (p < 0.05); #vs. stressed control (S) (p < 0.05).
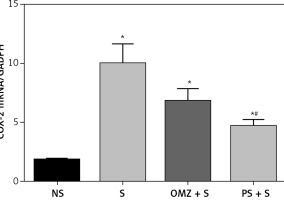
The mRNA for the COX-2 gene was low in the non-stressed rats but was significantly unregulated in all rats exposed to stress (Figure 6). The most significant increase was observed in the stressed control group (p = 0.0001). However, the COX-2 mRNA in the PS group was significantly lower (p = 0.01) when compared to the stressed control; this was not observed with OMZ supplementation.
Discussion
There are not many scientific reports regarding the effects of Piper sarmentosum, although it is widely used as an alternative medicine in Asian countries. When given orally, as in this study, Piper sarmentosum has been shown to be absorbed well from the gastrointestinal tract and to be well distributed to body tissues [16]. The results of the present study demonstrated that the methanolic extract of Piper sarmentosum had a protective effect on stress-induced gastric lesions, which may promote this herb as prophylaxis against gastric ulcer.
The results of this study demonstrated that pre-treatment of rats with Piper sarmentosum or omeprazole individually markedly reduced gastric mucosal damage induced by stress. The finding showed that rats exposed to water-immersion restraint stress for 3.5 h developed gastric mucosal lesions at the glandular part of the stomach. Pre-treatment with Piper sarmentosum and omeprazole reduced the lesion formation compared to the stressed control group. The lesion index however was comparable between these two treatment groups. The lesions were in the form of generalised erythema, haemorrhage and gastric erosion. There was a difference between these two agents in that a few rats in the Piper sarmentosum group developed lesions, while all the rats in the omeprazole-treated group developed stress-induced gastric injury, although at a lower magnitude compared to the stressed controls.
The gastric mucosa is continuously exposed to harmful factors, and the balance between the aggressive factors and protective capacity plays an integral role to maintain its functional integrity. Among various hazardous effects on biological systems, oxidative destruction of membrane polyunsaturated fatty acid, more commonly known as lipid peroxidation, has been observed in the gastric tissue [17, 18]. As shown in our previous studies [5, 19, 20] and in this study, the high gastric MDA content in the stressed control stomach supports the hypothesis that stress-induced injury is mediated by the lipid peroxidation process. This indicates that reactive oxygen species play an important role in the pathogenesis of gastric mucosal injury induced by stress. We also showed that Piper sarmentosum decreases the breakdown of the gastric mucosal barrier by reducing the product of lipid peroxidation. The reduced MDA level accompanied by the improved gastric lesions in this group suggests that Piper sarmentosum probably reduced gastric injury by retarding the lipid peroxidation process.
Piper sarmentosum possesses high antioxidant activity, and it might be attributed to the chemical components present in the plant such as vitamins C and E, xanthophylls, carotenes and phenols [10, 21, 22]. The plant has also been shown to contain a high amount of active naringenin, a potent antioxidant [23], which was approximately 87.6% [23]. This amount of naringenin was evidenced to reduce superoxide anion generation by up to 75.7%, making it a potent natural source of antioxidant [23]. This could be attributed to the ability of Piper sarmentosum to reduce oxidative stress, as shown in this study. Studies have also shown the ability of Piper sarmentosum to reduce oxidative stress in vivo [24, 25].
The findings also showed no difference in the ability to reduce gastric MDA content between Piper sarmentosum and omeprazole, suggesting similar radical scavenging ability. Omeprazole was previously shown to confer dose-dependent protection against acute gastric mucosal injury induced by ethanol by inhibiting lipid peroxidation and neutrophil infiltration significantly and also by preventing glutathione depletion [26]. Meanwhile, Biswas et al. [27] reported that omeprazole, apart from its well-known acid reducing ability, was able to block stress and indomethacin-induced gastric lesions through a mechanism independent of its role in acid secretion, which is by scavenging hydroxyl radicals.
Many investigators have suggested that acid has an important pathogenic role in stress-induced lesions due to the findings of increased acidity. This is because acid is generally accepted as an aggressive factor and may be responsible for mediating many forms of gastric lesions, as such investigators believe that under non-stress conditions the gastric mucosa is resistant to a high concentration of acid, that in stress the mucosal defence mechanism is weakened by extreme contraction of the gastric musculature and depletion of gastroprotective functions, and that a high amount of acid can cause significant damage to an impaired mucosa [28, 29].
The findings on gastric acidity changes in stress have been diverse. While some have found a reduction in gastric acidity [30, 31], many others have found that the increase in gastric acidity during stress plays an important role in inducing damage to the gastric mucosa [28, 32]. We found that the gastric acid concentration was increased in stressed rats, which was a similar finding to a previously described study. As predicted, omeprazole – a potent gastric acid secretion inhibitor – reduced the gastric acid concentration due to water-immersion restraint stress. Interestingly, we found that Piper sarmentosum was able to also reduce the gastric acid concentration, similar to the level of rats treated with omeprazole. Although it has been used traditionally to treat stomach ailments, to our knowledge this finding on the gastric acid reducing property has never been previously described. This effect of Piper sarmentosum on gastric acidity could be the reason behind its effectiveness to treat abdominal pain, gastric discomfort and gastritis in traditional use.
An important gastric defensive factor, PGE2, was found to decrease after exposure to stress. Prostaglandins are important in maintaining blood flow as well as mucus and bicarbonate to protect the gastric environment. Similar to our findings, other studies have showed that rats exposed to restraint stress had significant mucosal ulcerations, and this was associated with a significant decrease in the gastric mucosal levels of PGE2 [33, 34]. Brzozowski et al. [35] found that exposure to stress led to ischemia-reperfusion, which produced a significant fall in PGE2 generation in the gastric mucosa. Takeuchi et al. [36] also found that treatment with 16,16-dimethyl PGE2, was able to decrease the mucosal ulcer in rats exposed to stress. We also found that Piper sarmentosum supplementation has the ability to block the changes in PGE2, where the level was not significantly different to the non-stressed control group. With lower occurrence of lesions in the Piper sarmentosum supplemented groups with the maintenance of gastric PGE2 levels, we can also associate the decrease in the gastric lesions with the maintenance of the levels of PGE2.
Our findings as well as others suggests that PGE2 seems to be an important determinant in the pathogenesis of stress-induced gastric mucosal lesions. If this is true, then supplementation with Piper sarmentosum may be a good alternative for reducing stress-induced gastric lesions. However, supplementation with omeprazole failed to enhance PGE2 but was still able to decrease lipid peroxidation, as was found in this study. The difference in the protective mechanism between Piper sarmentosum and omeprazole as seen in this study opens the door for future research. Pharmacotherapy using misoprostol – a synthetic PGE1 analogue – showed a positive effect against stress-induced gastric injuries when administered at a high dose in patients in intensive care units (ICUs) [37]. Although effective, the side effects of diarrhoea and abdominal discomfort led to a low rate of misoprostol use in a clinical setting. The effectiveness of Piper sarmentosum in increasing PGE2 could account for its beneficial effect in reducing the risk of gastric ulcers in critically ill patients without the side effects of misoprostol. A previous study showed that oral ingestion of Piper sarmentosum at a very high dose of 2000 mg/kg did not show any subacute toxicity in rats [38].
COX-1 enzyme is important in providing the baseline or physiological PGE2 which helps maintain the mucus, bicarbonate and mucosal blood flow [18, 39–41]. Natale et al. [42] explained that in the presence of oxidative damage, the prostaglandins could be converted into products of oxidation such as 8-iso-PGF2α. In addition, oxidative stress could inhibit cyclooxygenase enzyme activity, thus reducing prostaglandin levels [43]. This however was not the case in this study; the COX-1 enzyme was not inhibited in stress. It was previously reported that COX-1 generation was observed in gastric mucosa under normal conditions and remained unchanged after exposure to stress [34], as shown in the current study. We can hypothesise that the loss of PGE2 due to stress is not via the down-regulation of COX-1 during stress but may be due to its conversion into oxidants [42] or due to the destruction of the lipid membrane which reduces the arachidonic acid – the precursor for prostaglandin synthesis. Research has also shown that stress induced accumulation of hydrogen peroxide (H2O2), a prostaglandin biosynthesis inhibitor [44], which could be the case in stress ulcers, where oxidative stress plays a major role in its pathogenesis. The effect of Piper sarmentosum on maintaining the gastric PGE2 level could be due to its antioxidant capabilities by increasing the level and activities of catalase and glutathione peroxidase, which reduce H2O2 [45, 46].
It is well known that prostaglandins produced by COX-1 play an essential role in maintaining the integrity of the gastric mucosa, while the PG fall produced by COX-2 is related to inflammation and destruction of the mucosa leading to the formation of gastric lesions [47, 48]. Thus providing the selective COX-2 inhibitor celecoxib significantly reduced the aggravated development of gastric lesions compared to non-selective NSAIDs. Interestingly, we found an increase in COX-2 mRNA expression in rats with gastric lesion formation. Brzozowski et al. [35] suggested that the expression of COX-2 mRNA after water-immersion restraint stress might be due to the deficiency in PGE2 generation in the gastric mucosa after stress. Thus, this expression might reflect the reduction of PGE2 content or activity. This enzyme is unregulated by pro-inflammatory cytokines and growth factors, where it mediates pathological reactions including inflammation [49, 50]. Research has shown the increase in pro-inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-1β in rats exposed to stress [51, 52]. It was also reported that COX-2 protein is highly localised in fibroblasts, monocytes/macrophages and granulocytes in the base of gastric ulcers in rats [53].
The findings in this study showed that stressed control rats had the highest rise in COX-2 expression compared to the rats treated with either omeprazole or Piper sarmentosum, and this correlated with the extent of damage to the gastric mucosa. The finding suggests that Piper sarmentosum also helps in protection of the gastric mucosa against stress-induced damage by blocking upregulation of COX-2 gene expression, which reduces the production of prostaglandins associated with inflammation. This finding was not observed in the rats pre-treated with omeprazole.
The reported incidence of stress-related mucosal damage varies from 6% to 100% in critically ill patients [54]. Morbidity and mortality of patients with critical illness are positively correlated with the degree of oxidative stress. Therefore, the administration of herbs with a high antioxidant content such as Piper sarmentosum seems to be a reasonable therapeutic approach. Enteral nutrition given early is now well established as part of therapy for the critically ill. Focus on evidence-based therapeutic nutrition is the future to enhance the nutrition algorithms as a new standard of care in the ICU. Antioxidants are functional nutrients that may prove to be beneficial as part of the enteral nutrition care for critical patients. This will provide micronutrient support which alongside pharmacotherapy may offer a fresh perspective towards the outcomes in critical patients.
In conclusion, this preliminary research showed the anti-ulcer properties of PS in preventing further damage to the gastric mucosa due to stress exposure. Our data suggest that stress-induced gastric injuries involve gastric lipid peroxidation, hyperacidity and reduction in the protective PGE2. Supplementation with Piper sarmentosum and omeprazole may be beneficial where their intake prevents the occurrence of gastric mucosal lesions by reducing damage by free radicals and gastric acid. Piper sarmentosum has the effect of restoring PGE2 gastric content which was altered by stress; this effect was not observed with omeprazole. These findings suggest that the methanolic extract of Piper sarmentosum possesses good gastroprotective properties which should be explored further. If found beneficial, supplementation with Piper sarmentosum may prove to be a good alternative for reducing stress-induced gastric ulcers in patients prone to developing ulcers and in critically ill patients.