Introduction
Breast cancer is the second most common malignancy worldwide, comprising 12% of all invasive cancers. It is the most common female malignancy, especially in well-developed countries. Breast cancer biology consists of an immense network of dependencies between various proteins. One of the widely discussed signaling proteins playing key roles in cancer development is osteopontin (OPN). It was described for the first time by Senger et al. (1979), who indicated its role as a potential marker of epithelial cell transformation [1]. The name of osteopontin is associated with its function in the bone tissue. Osteopontin binds to the cellular surface, enhancing cell migration and adhesion. Moreover, it plays a role in several physiological and pathological processes such as maintenance and remodeling of bone integrity in response to tension or pressure, early cell immune reactions, dystrophic calcification, recurrent coronary artery stenosis, regulation of growth and differentiation of cancer cells, and development of metastases. It has also been shown that osteopontin takes part in the process of new blood vessel formation. Furthermore, osteopontin is necessary in cell adhesion, apoptosis, inflammatory processes, and wound healing [2].
Current studies concentrate on establishing the role of osteopontin in carcinogenesis. It is suspected to influence the development of distant metastases in malignancies of the tongue, esophagus, stomach, colon, pancreas, kidneys, endometrium and breast; however, the mechanism remains unclear. Moreover, high OPN concentration in breast cancer cells has been shown to be correlated with sooner local relapse and shorter overall survival (OS) [3].
The molecular subtype of breast cancer that tends to metastasize more commonly than others is called triple-negative breast cancer (TNBC). It is defined by lack of expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). Triple-negative breast cancer is diagnosed in 12–17% of all breast cancer cases. As the TNBC cells are frequently relatively heterogeneous and poorly differentiated, this cancer type is thought to run an aggressive course with rapid recurrences [4] and common development of distant metastases [5].
Compared to other subtypes of invasive breast cancer, TNBC is characterized by higher clinical stages on diagnosis, larger size (pT1–pT4) and lower histological grade (G1–G3) [6]. Triple-negative breast cancer tends to be more aggressive, with rapid growth of the tumor, to metastasize both to regional lymph nodes and distant organs and to present a much shorter time to relapse after treatment (on average 1–3 years). Metastases of TNBC are localized more often in the brain and lungs, while localizing in the bones less commonly than other types of breast cancer [7, 8]. On the other hand, TNBC tend to have higher probability of a complete pathological response to neoadjuvant chemotherapy [9].
Due to more frequent occurrence of lymph node metastases in the case of TNBC and the same relationship between the presence of metastases and OPN expression, we decided to analyze the coexistence of loss of expression of breast cancer receptors (ER, PR, HER2) and expression of osteopontin.
The purpose of this study was to evaluate immunohistochemical expression of osteopontin in connection with the analysis of recognized clinical and pathological prognostic factors in primary sites of triple-negative breast cancer with and without lymph node metastases.
Material and methods
Material consisted of primary tumors of female breasts (726 patients) obtained from the excisional biopsies and total mastectomies. A total of 35 patients with triple-negative breast cancer confirmed by assessment of lack of the ER, PR and HER2 expression were selected for further testing. Tumor samples were fixed in 8% buffered formalin phosphate. After 24-hour fixation, material was dehydrated using alcohol in gradually increasing concentrations (50, 60, 50, 60, 70, 80, 90, 96%) of absolute alcohol and xylene and embedded in paraffin. Paraffin blocks were cut into serial sections 4 µm in thickness. They were then stained using standard methods. In preparations stained with H&E the following determinations were carried out:
type of neoplasm (WHO classification),
tumor grade (G1–G3) including tubule formation, and intensity of division as well as the degree of neoplastic cell differentiation,
mitotic index as the mean number of mitotic figures in neoplastic cells counted in 10 fields of vision at 400× magnification (surface area: 0.17 mm2).
Paraffin sections on slides covered with 2% saline solution (Sigma) in acetone and dried for 24 h at 42°C were used for immunohistochemical examination. Before the staining procedure, the material underwent a routine deparaffinization process in gradually decreasing concentrations of alcohol. For immunohistochemical examination we used monoclonal antibodies in proper concentrations in 1% BSA (Merck, Darmstadt, Germany). The following antibodies were used:
Monoclonal Mouse Anti-Human Estrogen Receptor alpha, 1 : 50 dilution, clone: 1D5, code: IR654 (DAKO, Santa Clara, United States),
Monoclonal Mouse Anti-Human Progesterone Receptor, 1 : 400 dilution, clone: PR636, code: IR068 (DAKO, Santa Clara, United States),
Polyclonal Rabbit Anti-Human HER2 Protein using HercepTest, code: K5204 (DAKO, Santa Clara, United States),
Mouse monoclonal OPN antibody (AKm2A1), sc-21742 (Santa Cruz Biotechnology, Dallas, Texas, United States).
Sections were subsequently dewaxed in xylene, gradually decreasing concentrations of alcohol and distilled water. The next step involved revealing the epitope by heating the slides in a buffer (derived from mixing 75 µl of blocking buffer with 5 ml PBS) for 1 h. Afterwards, preparations were incubated with 50–100 µl (depending on the sample size) of anti-osteopontin antibody 1 : 50 solution for 30 min. After incubation, preparations were rinsed three times in PBS for 5 min. The next step was incubation for 30 min with biotinylated secondary antibody (derived from mixing 75 µl of blocking buffer with 5 ml of PBS and 25 µl of biotinylated secondary antibody). After incubation, preparations were once again rinsed three times in PBS for 5 min. Next, preparations were incubated for 30 min with an AB enzyme reagent that was previously prepared by combining avidin with biotinylated peroxidase in an equal ratio and diluted in PBS (50 µl of avidin, 50 µl of biotinylated peroxidase and 2.5 ml of PBS). After incubation, preparations were rinsed three times in PBS for 5 min. The next step was incubation in a peroxidase substrate (consisting of 2.5 ml of distilled water, 5 drops of buffer, 1 drop of diaminobenzidine solution (DAB) and 1 drop of peroxidase) for about 5 min. The color of the preparations was controlled, then they were rinsed in tap water, stained for 5 min with Ehrlich’s hematoxylin, differentiated in 1% acidic alcohol and again rinsed in tap water. The preparations were then dehydrated in a series of alcohols with increasing concentrations and xylene and mounted in a DPX mountant (Merck, Darmstadt, Germany).
To interpret the results of immunohistochemical (IHC) staining computer image analysis and the program Lucia v. 4.21 were used. Stained nuclei, cytoplasm and cellular membrane of cancer cells were counted among 1000 cancer cells. OPN staining results were scored according to the percentage of positive cells as follows:
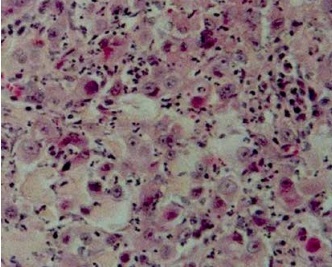
Figure 1
Histopathological image of triple-negative breast cancer (grade 3, H&E, original magnification, 200×)
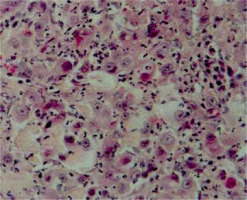
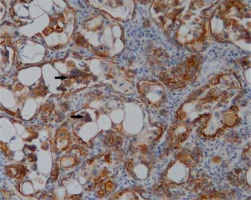
Figure 2
Immunohistochemical analysis of osteopontin expression in triple-negative breast cancer (positive staining images for OPN; original magnification, 400×)
Statistical analysis
The results were presented in the form of arithmetic means (x ± SD). The results were developed using the SPSS v. 12.0 PL Windows program. Pearson’s quadrate test was used to verify the hypothesis of the independence of two features. Spearman’s correlation coefficient values were calculated, and their significance was evaluated using Student’s t-test. Differences were considered statistically significant at p ≤ 0.05.
Results
In our study patients with TNBC represented 4.8% of cases. Mean age of studied women was 57.1 years. Histological examination revealed the following percentages of TNBC tumors types: 89% – invasive ductal carcinomas (IDC) and 11% – metaplastic carcinomas.
In the TNBC group, tumors evaluated as G2 (51%) and G3 (42.9%) were the most numerous. As far as tumor size is concerned, the most numerous group was represented by pT2 (2–5 cm) (57.1% of cases), whereas 26% of tumors were below 2 cm in diameter, so they were evaluated as pT1. Remaining patients were diagnosed with tumors exceeding 5 cm, and additionally 2 malignancies (6%) infiltrated the chest (pT4). Lymph node involvement was detected in 17 cases (49%) of TNBC (pN1 43%; pN2 3%; pN3 3%). The correlation between nodal status (pN0–pN3), histological grade (G1–G3) and tumor size (pT1–pT4) is presented in Table I.
Table I
Relationship between presence of nodal metastases (pN0–pN3), histological grade (G1–G3) and tumor size (pT1–pT4) in triple-negative breast cancer
In our study G1 tumors did not present lymph node involvement and their measurement was below 5 cm. A statistically significant relationship was demonstrated between lymph node involvement and tumor size (p-value ≤ 0.05).
Age of examined patients with TNBC was within the range of 30 to 81. Although the correlation between patient age and histological type of neoplasm was studied and no statistically significant relationships were found, IDC was most frequently examined in patients in the age range of 45 to 65. In our study a statistically significant correlation was demonstrated between histological grade (G1–G3) and tumor size (pT1–pT4) (p ≤ 0.044), as well as between lymph node involvement (pN0–pN3) and tumor size of TNBC malignancies (p ≤ 0.049).
Moreover, in our analysis we assessed the relationship between patients’ age and histological grade (G1–G3) and noted that young women were diagnosed with G1 breast cancer more frequently.
Analysis of the structure of increasing histological grading matched with patient age revealed that 66.7% of women at the age between 60 and 75 were diagnosed with G2 tumors, whereas patients over 75 years of age were diagnosed most frequently with G3 tumors – also 66.7% of cases. Interestingly, none of the patients over 60 years was diagnosed with the lowest grading, G1. To conclude, there is an evident increase of histological grading with patient age.
Through the use of immunohistochemical techniques, expression of osteopontin was detected in 35 cases of TNBC. OPN staining results were scored according to the percentage of positive cytoplasm staining based on the scale presented in Table II. In the studied group of 35 patients with TNBC all of them revealed OPN expression, mostly scored as [+] (57.1%) and [++] (42.9%). In the studied materials expression scored as [+++] was not detected.
Table II
Scale of assessment of immunohistochemical staining for osteopontin in patients with triple-negative breast cancer
In our study we assessed the correlation between expression of osteopontin and recognized clinical and pathological prognostic factors used in breast cancer diagnostics such as histological grade (G1–G3), tumor size (pT1–pT4), lymph node involvement (pN0–pN3) and expression of steroid receptors (ER, PR) and HER2. Among studied factors we highlighted the correlation between lymph node involvement and osteopontin expression (p ≤ 0.001) (Table III). Nodal metastases were detected in 93% of patients with OPN expression scored as [++] (> 10–30% positive cells), whereas only 15% of patients with OPN expression scored as [+] (10% positive cells) revealed lymph node metastases (Table III). Given grading, tumor size and patient age, no statistically significant relation between them and osteopontin expression in sites of TNBC was detected (Tables III and IV).
Table III
Relationship between osteopontin expression and histological grade (G1–G3), tumor size (pT1–pT4), presence of nodal metastases (pN0–pN3)
Table IV
Correlation between osteopontin expression and histological grade (G1–G3), tumor size (pT1–pT4), presence of nodal metastases (pN0–pN3) in patients with triple-negative breast cancer
| Variable | Histological grade (G1–G3) | Tumor size (pT1–pT4) | Nodal status (pN0–pN3) | Patient age [years] | ||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | |
| Osteopontin expression | –0.055 | 0.754 | –0.074 | 0.674 | 0.776 | < 0.001 | –0.208 | 0.236 |
Discussion
Breast cancer is the second most common malignancy worldwide, right after lung cancer. Among women it is the most commonly diagnosed malignant tumor, causing 13% of deaths. Triple-negative breast cancer represents the least numerous group of breast cancers among women (12–20%). Due to the rarity of TNBC, our study sample was not very extensive, including only 35 cases, demanding cautious interpretation of the results, which is a common problem in papers concerning infrequent malignancies. As this type of breast cancer is characterized by the worst prognosis, it seems crucial to find new markers connected with well-established prognostic factors such as: expression of estrogen, progesterone and HER2 receptors, histological grading, tumor size and nodal status [7, 10]. These findings may influence selection of effective therapy for TNBC in the future.
For the time being researchers are looking for new prognostic and predictive markers for TNBC, and osteopontin might fulfill this role. It has been widely studied not only regarding breast cancer, but also non-small-cell lung carcinoma (NSCLC), gastric cancer, liver cancer, head and neck cancers and prostate cancer.
In our study mean age of studied patients was 57 and similar results were obtained by Wang-Brown et al. (2015) (mean age 58), whereas slightly different results were achieved by other researchers, e.g. Bhoo-Pathy et al. (2015) and Dent et al. (2007) (mean age 53) and also Nabi et al. (2016) (mean age 47) [11–13].
Given the tumor size, the largest group of triple-negative breast cancer encompassed tumor assessed as pT2 (57.1%), whereas 26% of tumors were below 2 cm. Remaining patients were diagnosed with tumors exceeding 5 cm, while 2 malignancies (6%) infiltrated the chest (pT4). Dent et al. (2007) and Badowska-Kozakiewicz et al. (2018) obtained similar results – they confirmed in their study that pT2 cancers (accordingly: 55.6% and 62.1%) comprised the largest group of triple-negative neoplasms, and also Fan et al. (2015) reported that among studied TNBC patients, 50% were diagnosed with pT2 tumors [13–15].
In our study lymph nodes metastases were detected in 49% of patients with TNBC (43% – pN1; 3% – pN2; 3% – pN3). Similar results were reported by Fan et al. (2015) and Hernandez-Aya et al. (2011) – they confirmed that the majority of their patients did not demonstrate lymph node involvement (57.5% and 54%). However, as opposed to our analysis, both research groups observed a greater percentage of pN2 and pN3 (pN2 – 9.5%, pN3 – 8.3%; pN2 – 8.7%; pN3 – 6.1%). Studies conducted by Dent et al. (2007) demonstrated distinctive results with the majority of TNBC patients presenting nodal metastases [13, 15, 16].
In all examined triple-negative tumors histological grading was assessed and 6% of them were stated as G1, 51% G2 and 43% G3. Fan et al. (2015) obtained similar results, assessing 45.7% of cancer in the TNBC group as G3. A much higher percentage of G3 patients with triple-negative tumors was reported by Nabi et al. (2016) and Rambau et al. (2014) (56%; 85%) [15, 17, 18].
Osteopontin is proved to be a specific phosphorylated glycoprotein playing a key role in many physiological processes such as osteoclastogenesis, angiogenesis and cell division [19]. In the past records higher expression of osteopontin was determined in cancer tissues, in comparison to healthy ones [3]. Recent studied confirmed that a higher level of osteopontin expression correlates with faster relapse and shorter OS [3].
The main aim of our study was to evaluate the correlation between expression of osteopontin and patient age, histological grading, tumor size and lymph node metastases. Our analysis revealed a statistically significant association between osteopontin expression and lymph node involvement – it could suggest that osteopontin may become a new marker of triple-negative breast cancer invasiveness. In our study an average level [++] of osteopontin expression was noted in 82% of patients with TNBC and lymph nodes metastases, whereas the same level of osteopontin expression was found only in 6% of patients without nodal involvement. However, it is worth pointing out that OPN was expressed in all TNBC cases and it was the level of expression that correlated with lymph node metastases, rather than expression itself. This indicates that OPN might be an unspecific marker. Moreover, our study lacks information about the clinical course of the patients whose tissue samples were included, preventing us from expanding our analysis to whether the increased occurrence of lymph node metastases influences the clinical outcome. Allan et al. (2006) and Rudland et al. (2002) reported similar results, finding higher expression of osteopontin in tumors obtained from patients with nodal metastases, but it had no statistical significance (p = 0.69; p = 0.17) [20, 21]. Rudland et al. (2002) confirmed the association between osteopontin expression and level of histological grading (G1–G3). They assessed 30% of OPN-positive tumors as G3 in comparison to 17% of those that were without osteopontin expression. Likewise, their study demonstrated that an increased level of osteopontin is associated with decreased disease-free survival (DFS) and OS of the patients. Bramwell et al. (2014), Xu et al. (2015) and Thorat et al. (2013) studied the correlation between OPN and grading, tumor size and nodal involvement but they did not obtain any statistically significant results [20–24]. In their research Marcil et al. (2009) revealed OPN-positive tumors in 93% of patients with nodal metastases, whereas only 75% of patients without nodal involvement showed expression of osteopontin. Sun et al. (2012) and Hu et al. (2005) obtained similar results, stating that in patients with metastasis to lymph nodes OPN-positive tumors occur more frequently. There are conflicting reports regarding the association between osteopontin expression and grading. Marcil et al. (2009) and Rud et al. (2012) found a positive correlation between given factors, whereas Boldrini et al. (2005) and Donati et al. (2005) found this correlation negative [19, 25–29].
Weber et al. (2010) conducted a meta-analysis of 228 publications about osteopontin and its role as a marker for cancer aggressiveness and patient survival. After carrying out a comprehensive analysis they concluded that osteopontin is a valuable marker for patient survival concerning breast cancer, prostate cancer, head and neck cancers and liver cancer. Patients with OPN-positive tumors had lower overall and relapse-free survival. Weber et al. (2010) also confirmed the association between higher expression of osteopontin and lymph node metastases in eight cancers, including breast, lung, head and neck, gastric and esophageal cancer [30].
Various studies concentrate on mechanisms through which increased OPN expression promotes cancer progression, as described above. It appears that OPN plays important stimulating roles at every step of tumor aggressiveness: inducing cell proliferation [31], angiogenesis [32], mediating epithelia-mesenchymal transition [33] and, thus, development of distant metastases [34].
Studies concerning the structure of the OPN gene identified several enhancers enabling different transcriptional factors to influence the intensification of OPN gene transcription [35]. Among those is activator protein-1 (AP-1), a complex hetero- or homodimer protein of Jun and Fos oncogenic protein families [36]. Its function is currently being studied in various neoplasms, including triple-negative breast cancer, where it was shown to facilitate the progression of the tumor, for example by suppressing the expression of E-cadherin, decreasing cell-to-cell adhesion [37]. It would be worth considering the possibility of expression of OPN as a marker of AP-1 activation, which itself facilitates metastatic spread of the disease. Description of the Ras-activated enhancer in the promoter of the OPN gene [38] indicates that OPN expression might also be upregulated due to Ras oncogene activity, which is infrequent, but present in some cases of triple-negative breast cancer [39]. This suggests that increased expression of OPN observed in triple-negative breast cancer may result from enhanced transcription due to presence of oncogenic transcription factors. However, this matter requires detailed research.
Xu et al. (2016) conducted a study on mixed cell co-cultures of mammary fibroblasts with altered T-lymphoma invasion and metastasis-inducing protein 1 (Tiam1) expression and breast cancer cells. Decreased Tiam1 expression in fibroblasts resulted in increased secretion of osteopontin, leading to long-term increase of invasiveness in associated human breast cancer lines. The authors concluded that the Tiam1-OPN signaling pathway may regulate breast cancer invasiveness by causing epigenetic alterations of breast cancer stem cells and could be a potential therapeutic target [40].
Pio et al. (2017) studied ALDHhiCD44+CD24- breast cancer cell migration in bone marrow-conditioned media in vitro. Their results showed that bone-derived OPN increased the migration and promoted maintenance of the stem-like behavior of breast cancer cells. They also demonstrated that the mechanism of this dependence is mediated by interactions between OPN and CD-44 and RGD-dependent integrins. In the studied cells, bone-derived OPN activated the downstream signaling pathway involving phosphorylation of WNK-1 and PRAS40 [41].
Osteopontin is not only correlated with invasiveness but also is suspected to be associated with the response to chemotherapy. OPN expression is always correlated with vasculogenic mimicry (VM) – a new model of neovascularization based on a microvascular channel made up of non-endothelial cells [42]. In the Gu et al. (2017) study, 80 out of 200 analyzed breast cancer samples demonstrated positive expression of OPN. They proved that concurrence of OPN expression and VM might be used as a predictive factor for efficiency of neoadjuvant chemotherapy due to the significant correlation between its expression and pathological complete response [43]. The addition of one splice variant of OPN (exon-4) is suggested to be used in standard breast cancer immunohistochemistry owing to its potential in making decisions regarding the way of treatment. In the study conducted by Zduniak et al. (2016) osteopontin exon 4 was associated with a favorable response to tamoxifen and a poor response to CMF chemotherapy (cyclophosphamide, methotrexate, fluorouracil) [44]. What is more, high expression of OPN mRNA is correlated with reduced DFS and OS [45].
In conclusion, our study confirmed that invasive ductal carcinoma comprises the most numerous groups among all histological types of breast cancer. Furthermore, the analysis conducted on patients with triple-negative breast cancer confirmed the association between nodal metastases (pN0–pN3) and tumor size (pT1–pT4) and highlighted the statistically significant correlation between osteopontin expression and lymph node involvement, which may suggest osteopontin’s important role in the invasiveness of triple-negative breast cancer.