Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
Identifying the causal relationship between gut microbiota and lymphoma disease: a Mendelian randomization study and meta-analysis
1
Changsha Medical University, Changsha, China
2
The First Clinical College, Changsha Medical University, Changsha, China
3
Department of Basic Medical Sciences, Changsha Medical University, Changsha, Hunan, China
4
Hunan Provincial University Key Laboratory of the Fundamental and Clinical Research on Functional Nucleic Acid
Submission date: 2024-08-15
Final revision date: 2024-12-11
Acceptance date: 2024-12-28
Online publication date: 2025-02-18
Corresponding author
Danna Chen
Department of Basic Medical Sciences Changsha Medical University 1501 Leifeng St Changsha, Hunan 410219, China, Phone: +86-731-84805339, Fax: +86–731-84 478 152
Department of Basic Medical Sciences Changsha Medical University 1501 Leifeng St Changsha, Hunan 410219, China, Phone: +86-731-84805339, Fax: +86–731-84 478 152
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Increasing evidence from observational studies and clinical trials suggests that gut microbiota (GM) are associated with lymphoma. However, it is unclear whether there is a causal relationship between GM and lymphoma.
Material and methods:
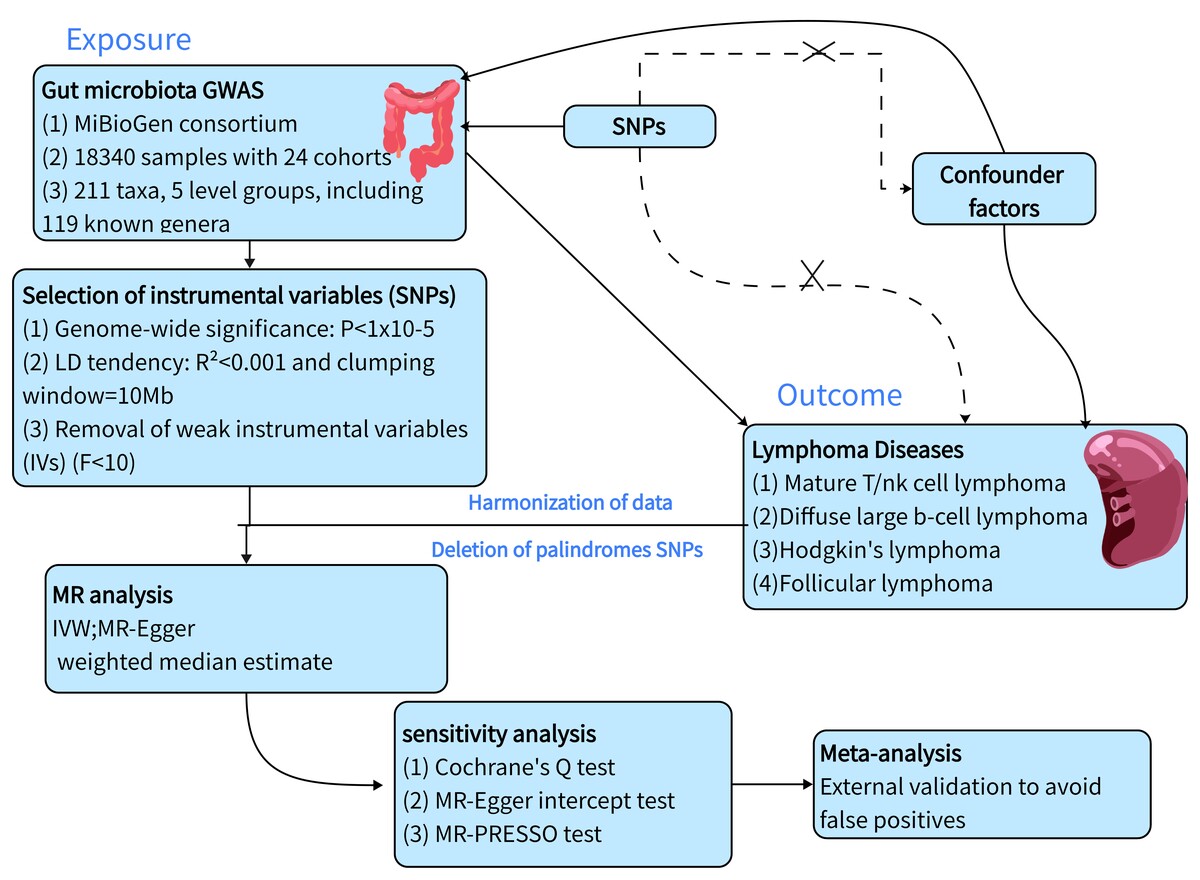
To evaluate the causal relationship between GM and lymphoma, we conducted a two-sample Mendelian randomization analysis based on the genome-wide association study (GWAS). GWAS summary statistics of the GM were obtained from the MiBioGen study that including 211 taxa. GWAS summary statistics of four lymphomas were obtained from the IEU Open GWAS study. Then, we systematically performed sensitivity analyses and heterogeneity analysis to verify the reliability of our findings. Finally, we used an external cohort for validation and performed a meta-analysis of the positive results.
Results:
We identified 37 causal relationships between GM and lymphoma. The results of the sensitivity analyses demonstrated the reliability of the MR analysis. Furthermore, the combined meta-analysis yielded nine significant results, with the most notable being Terrisporobacter (MetaOR = 2.39, 95% CI = 1.07–5.32, p = 0.03), which was identified as a pathogenic factor for diffuse large B-cell lymphoma (DLBCL). Methanobrevibacter (MetaOR = 0.49, 95% CI = 0.27–0.92, p = 0.03) was identified as a protective factor against DLBCL. Cyanobacteria (MetaOR = 1.94, 95% CI = 1.24–3.03, p = 0.004) were identified as a pathogenic factor for FL.
Conclusions:
Our study identified a causal relationship between gut microbes and four lymphoma diseases. To further confirm the causal relationship, external validation was performed, thereby providing new insights into the subsequent mechanisms by which gut microbes mediate lymphoma development.
Increasing evidence from observational studies and clinical trials suggests that gut microbiota (GM) are associated with lymphoma. However, it is unclear whether there is a causal relationship between GM and lymphoma.
Material and methods:
To evaluate the causal relationship between GM and lymphoma, we conducted a two-sample Mendelian randomization analysis based on the genome-wide association study (GWAS). GWAS summary statistics of the GM were obtained from the MiBioGen study that including 211 taxa. GWAS summary statistics of four lymphomas were obtained from the IEU Open GWAS study. Then, we systematically performed sensitivity analyses and heterogeneity analysis to verify the reliability of our findings. Finally, we used an external cohort for validation and performed a meta-analysis of the positive results.
Results:
We identified 37 causal relationships between GM and lymphoma. The results of the sensitivity analyses demonstrated the reliability of the MR analysis. Furthermore, the combined meta-analysis yielded nine significant results, with the most notable being Terrisporobacter (MetaOR = 2.39, 95% CI = 1.07–5.32, p = 0.03), which was identified as a pathogenic factor for diffuse large B-cell lymphoma (DLBCL). Methanobrevibacter (MetaOR = 0.49, 95% CI = 0.27–0.92, p = 0.03) was identified as a protective factor against DLBCL. Cyanobacteria (MetaOR = 1.94, 95% CI = 1.24–3.03, p = 0.004) were identified as a pathogenic factor for FL.
Conclusions:
Our study identified a causal relationship between gut microbes and four lymphoma diseases. To further confirm the causal relationship, external validation was performed, thereby providing new insights into the subsequent mechanisms by which gut microbes mediate lymphoma development.
REFERENCES (42)
1.
Yıldırım M, Kaya V, Demirpençe Ö, Paydaş S. The role of gender in patients with diffuse large b cell lymphoma treated with rituximab-containing regimens: a meta-analysis. Arch Med Sci 2015; 11: 708-14.
2.
Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet 2017; 390: 298-310.
4.
Berndt SI, Vijai J, Benavente Y, et al. Distinct germline genetic susceptibility profiles identified for common non-Hodgkin lymphoma subtypes. Leukemia 2022; 36: 2835-44.
5.
Ansell SM. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc 2015; 90: 1152-63.
6.
Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, Chang MW. Microbiome and human health: current understanding, engineering, and enabling technologies. Chem Rev 2023; 123: 31-72.
7.
Su YN, Wang MJ, Yang JP, et al. Effects of yulin tong bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front Endocrinol 2023; 14: 1122709.
8.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut 2022; 71: 1020-32.
9.
Liu Y, Li H, Wang X, et al. Anti-Alzheimers molecular mechanism of icariin: insights from gut microbiota, metabolomics, and network pharmacology. J Transl Med 2023; 21: 277.
10.
Scott AJ, Alexander JL, Merrifield CA, et al. International cancer microbiome consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019; 68: 1624-32.
11.
Wang K, Ma J, Li Y, et al. Corrigendum: Effects of essential oil extracted from artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front Nutr 2024; 11: 1416210.
12.
Murdaca G, Gerosa A, Paladin F, Petrocchi L, Banchero S, Gangemi S. Vitamin D and microbiota: is there a link with allergies? Int J Mol Sci 2021; 22: 4288.
13.
Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med 2011; 6: 487-95.
14.
Peppas I, Ford AM, Furness CL, Greaves MF. Gut microbiome immaturity and childhood acute lymphoblastic leukaemia. Nat Rev Cancer 2023; 23: 565-76.
15.
Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin 2017; 67: 326-44..
16.
Hu Y, Li J, Ni F, et al. Car-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun 2022; 13: 5313.
17.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008; 27: 1133-63.
18.
Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using gwas summary data. PLoS Genet 2017; 13: e1007081.
19.
Burgess S, Thompson SG. Bias in causal estimates from mendelian randomization studies with weak instruments. Stat Med 2011; 30: 1312-23.
20.
Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Primers 2022; 2: 6.
21.
Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019; 68: 1417-29.
22.
Delannoy-Bruno O, Desai C, Raman AS, et al. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature 2021; 595: 91-5.
23.
Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet 2021; 53: 156-65.
24.
Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32: 1-22.
25.
Myers TA, Chanock SJ, Machiela MJ. Ldlinkr: An R Package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front Genet 2020; 11: 157.
26.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50: 693-8.
27.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016; 40: 304-14.
28.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the Mr-Egger method. Eur J Epidemiol 2017; 32: 377-89.
29.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013; 37: 658-65..
30.
Sadik A, Dardani C, Pagoni P, et al. Parental inflammatory bowel disease and autism in children. Nat Med 2022; 28: 1406-11..
31.
Lu H, Xu X, Fu D, et al. Butyrate-producing eubacterium rectale suppresses lymphomagenesis by alleviating the Tnf-Induced Tlr4/Myd88/Nf-Κb Axis. Cell Host Microbe 2022; 30: 1139-50.e7.
32.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014; 12: 661-72.
33.
Ang Z, Ding JL. Gpr41 and Gpr43 in obesity and inflammation - protective or causative? Front Immunol 2016; 7: 28.
34.
Buda A, Qualtrough D, Jepson MA, Martines D, Paraskeva C, Pignatelli M. Butyrate downregulates alpha2beta1 integrin: a possible role in the induction of apoptosis in colorectal cancer cell lines. Gut 2003; 52: 729-34.
35.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016; 16: 341-52.
36.
Cheng MP, Domingo MC, Lévesque S, Yansouni CP. A case report of a deep surgical site infection with Terrisporobacter glycolicus/T. mayombei and review of the literature. BMC Infect Dis 2016; 16: 529.
37.
Chen JH, Zeng LY, Zhao YF, et al. Causal effects of gut microbiota on sepsis: a two-sample mendelian randomization study. Front Microbiol 2023; 14: 1167416.
38.
Jiang X, Zhang H, Zhang H, et al. Microcystin-Lr-induced interaction between M2 tumor-associated macrophage and colorectal cancer cell promotes colorectal cancer cell migration through regulating the expression of Tgf-Β1 and Cst3. Int J Mol Sci 2023; 24: 10527.
39.
Liu W, Wang L, Yang X, et al. Environmental microcystin exposure increases liver injury risk induced by hepatitis B virus combined with aflatoxin: a cross-sectional study in Southwest China. Environ Sci Technol 2017; 51: 6367-78.
40.
Martínez Hernández J, López-Rodas V, Costas E. Microcystins from tap water could be a risk factor for liver and colorectal cancer: a risk intensified by global change. Med Hypotheses 2009; 72: 539-40.
41.
Xu P, Zhang XX, Miao C, et al. Promotion of melanoma cell invasion and tumor metastasis by microcystin-Lr via phosphatidylinositol 3-kinase/Akt pathway. Environ Sci Technol 2013; 47: 8801-8.
42.
Shi Z, Hu G, Li MW, et al. Gut microbiota as non-invasive diagnostic and prognostic biomarkers for natural killer/T-cell lymphoma. Gut 2023; 72: 1999-2002.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.