A 41-year-old male patient, diagnosed with ankylosing spondylitis for 14 years, had intermittently taken various non-steroidal anti-inflammatory drugs, with poor efficacy. He used etanercept from September 2023 to March 2024 but discontinued it due to an inadequate therapeutic effect. On April 12, 2024, he was treated with AK111, a secukinumab biosimilar, 150 mg every 4 weeks subcutaneously (including a 1-month induction period), which effectively controlled his condition.
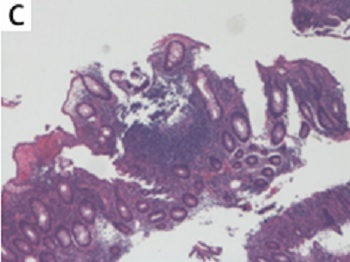
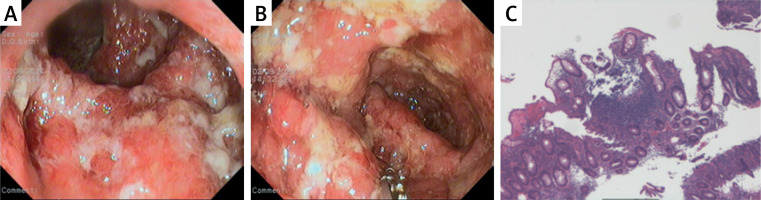
In early June 2024, the patient developed symmetrical erythema nodosum on the anterior tibial region of both lower limbs with tenderness, which regressed after an intramuscular injection of 1 ml of compound betamethasone. In early August 2024, the patient began to experience mucopurulent bloody stools, 7–8 times a day, accompanied by a significant sensation of tenesmus, and intermittent dull pain in the left lower abdomen, and his weight had decreased by 10 kg over the past 4 months, but he did not seek medical attention in a timely manner. On August 30, 2024, the patient returned to the hospital for examination, and fecal occult blood showed that red blood cells were 5–30 per high-power field (HP) and white blood cells were 23–53 per HP. Erythrocyte sedimentation rate (ESR) was 29 mm/h (0–15 mm/h), and C-reactive protein (CRP) was 59.9 mg/l (0–6 mg/l). Stool bacterial culture, Clostridium difficile toxin testing, and quantitative detection of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) DNA were all negative, and hemoglobin had decreased by 48 g/l over the past 4 months. On September 2, 2024, enteroscopy revealed diffuse and continuous congestion, swelling, and erosion of the mucosa in the sigmoid colon and rectum, with multiple ulcers covered by a large amount of purulent white exudate (Figures 1 A and B). Biopsy of the rectal and sigmoid colon mucosa showed a large number of acute and chronic inflammatory cells infiltrating the lamina propria, with focal areas of fibrous tissue, vascular proliferation, and inflammatory exudates, and an immunohistochemistry test for cytomegalovirus was negative (Figure 1 C). The patient was diagnosed with ulcerative colitis with a Mayo score of 10. On September 3, 2024, the treatment with the secukinumab biosimilar was discontinued, and the patient was given methylprednisolone 40 mg intravenous drip qd and mesalazine 1 g qid for induction of remission, but the symptom control was not suboptimal. On September 9, 2024, upadacitinib 45 mg qd was added, and methylprednisolone was discontinued on September 11, 2024. By September 13, 2024, the patient’s gastrointestinal symptoms had significantly improved, and he was discharged from the hospital.
Figure 1
A, B – multiple ulcers and purulent white exudate in the sigmoid colon and rectum. C – A large number of acute and chronic inflammatory cells infiltrating the lamina propria
The role of type 17 immunity in the pathogenesis of inflammatory bowel disease (IBD) is complex and characterized by conflicting findings. Interestingly, blockade of both IL-17A and IL-17F ameliorates colitis, while inhibition of only IL-17A is insufficient. Blocking IL-17A may even exacerbate colitis, while perturbation of IL-17F has been reported to reduce disease activity in a DSS-induced colitis model. This dichotomy, despite both IL-17A and IL-17F acting on the same receptor, implies that each may play distinct roles in the initiation and progression of colitis [1].
The patient has no family history of IBD, and previously had no symptoms related to chronic gastrointestinal diseases. After using a biosimilar of secukinumab, he first developed erythema nodosum, then newly developed ulcerative colitis, which is consistent with the general pattern reported in the literature that IL-17A inhibitors can induce IBD [2, 3]: the median time for the appearance of new IBD symptoms is 4.0 (1.5–7.5) months; he had previously been treated with etanercept; after discontinuing the IL-17A inhibitor and treating IBD, the gastrointestinal symptoms were significantly relieved. Erythema nodosum is also a common extraintestinal manifestation of ulcerative colitis. At that time, the patient lacked gastrointestinal symptoms and did not undergo in-depth screening. The glucocorticoids used to treat erythema nodosum may have delayed the outbreak of ulcerative colitis to some extent. Patients treated with secukinumab are at risk of developing IBD, and this phenomenon can be explained by two possibilities. One is that IL-17 inhibitors induce new IBD. In T-cell-deficient mice lacking IL-17A, more aggressive colitis has been observed [4]; the other possibility is that IL-17A inhibitors cannot control the progression of the disease in patients with subclinical IBD that has already appeared, eventually developing into confirmed IBD [5]. The cause of IBD in this patient is more likely to be the former.
Secukinumab biosimilars, like the original drug, carry the risk of inducing IBD. Clinicians should carefully assess and closely monitor the risk of IBD when prescribing IL-17A inhibitors. When nodular erythema or other clinical clues suggesting IBD appear, active screening, such as comprehensive patient history, fecal calprotectin or even colonoscopy is very necessary.