Hemichorea-hemiballismus can be caused by anatomical, neurochemical, or metabolic problems with the basal ganglia [1]. Hyperglycemia-induced hemiballismus is more common, while hypoglycemia-induced movement disorders have been recorded less frequently [1–3]. In movement disorders induced by hypoglycemia, the development of hyperintense lesions in the contralateral putamen and striatal areas on MRI-T1 imaging studies is the typical feature [4, 5]. We describe a case of hemichorea-hemiballismus in which T1 imaging was normal throughout a severe and persistent hypoglycemic episode, but diffusion-weighted imaging (DWI) revealed a hyperintense lesion in the left lentiform and caudate nuclei.
For 3 days, a 69-year-old female patient with hypertension and type 2 diabetes mellitus (DM) had complained of involuntary movements in her right arms and legs at the emergency room. She was conscious during her neurological evaluation, and right-sided hemichorea-hemiballismus was seen. Apart from DM, her medical and family histories were normal. The blood glucose level was 28 mg/dl and the urea level was 47 mg/dl. Hypoglycemia and metabolic acidosis showed no signs or symptoms. Blood glucose levels at home were reported to range between 20 and 50 mg/dl, for 3 days. Glycated hemoglobin (HbA1c) level was 8.3%. Her family had no history of any movement disorder, such as Sydenham’s chorea or Huntington’s chorea. Collagenous tissue diseases were excluded. The patient was given 20% dextrose liquids and admitted to the neurology department. The patient was using 10 units of long-acting insulin glargine once a day, 18– 20 units of short-acting regular insulin twice a day, 10 mg of amlodipine, and 100/25 mg of losartan/hydrochlorothiazide combination as antihypertensive treatment, and these drugs caused a movement disorder as a side effect which had not been reported. Upon admission, hyperintensity was seen in the left lentiform nucleus and caudate nucleus on DWI (Figure 1). The blood sugar was controlled and the movement disorder was treated with 5 mg of olanzapine. DWI and T2 FLAIR-weighted imaging revealed a lesion consistent with subacute ischemia in the head of the left caudate nucleus 2 days after admission, but T1 imaging was also normal (Figures 2, 3). The large vascular blockage was not seen on contrast-enhanced cerebral and cervical angiography, and plaque development was not seen. On the fourth day of therapy, the blood glucose level was approximately 150 mg/dl, and hemichorea-hemiballistic movements were under control.
Figure 1
On diffusion-weighted imaging in the left lentiform nucleus, caudate nucleus, and anterior leg of the internal capsule hyperintense lesion (A), and on ADC-weighted imaging isointense lesions (B) were observed
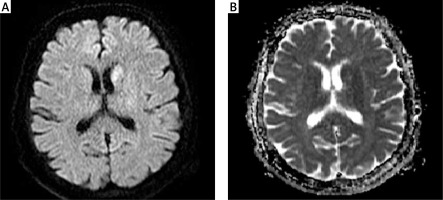
Figure 2
Two days after admission a: on diffusion-weighted imaging in the left lentiform nucleus, caudate nucleus, and anterior leg of the internal capsule hyperintense lesion (A), on ADC-weighted imaging hypointense lesions (B), and T1-weighted examination isointense lesions (C) were observed
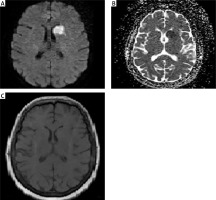
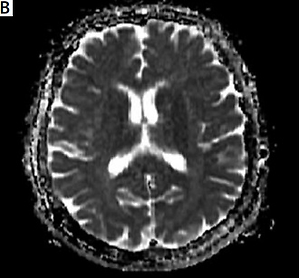
Figure 3
Two days after admission, on gradientweighted imaging in the left lentiform nucleus, caudate nucleus, and anterior leg of the internal capsule a hyperintense lesion (A), on T2-weighted imaging hypointense lesions (B), and on T2-FLAIR weighted examination hyperintense lesions (C) were observed
Various explanations for the pathophysiology underlying hypoglycemia-induced movement disorders have been suggested. The first is that it causes brain damage through neuroinflammatory pathways, resulting in permanent ischemia [6]. The second explanation is that a drop in blood glucose levels causes a drop in pyruvate levels and a division in the Krebs cycle, resulting in greater utilization of α-ketoglutarate and an increase in aspartate, an excitatory amino acid, leading to a movement disorder [6]. The third explanation is that the basal ganglia and related areas, which are sensitized by hyperglycemia, become more sensitive to hypoglycemia, and that the levels of GABA and acetylcholine used as alternative energy sources decrease during the hyperglycemic period, facilitating the formation of a sensitive environment during the subsequent hypoglycemia period [4, 5]. The fourth explanation is that hypoglycemia produces a drop in nitric oxide levels by disturbing the fibrinolytic balance and boosting platelet activation and vascular adhesion factors [6].
The damage induced by hypoglycemia is not restricted to specific locations, and lesions can grow in other regions via the brain’s connections. For example, in an 89-year-old diabetic patient who had hemiballistic movements, a transitory ischemic lesion forming in the posterior leg of the internal capsule without any lesion in the basal ganglia was seen and a temporary pyramidal tract injury was blamed for the movement problem [2]. In another patient, who exhibited choreoathetotic movements as a result of recurrent hypoglycemia, T1-weighted imaging revealed a hyperintense lesion in the putaminal area. Despite normal blood glucose levels and a normal cerebral MRI, recurring choreoathetotic movements were noted following the same patient’s femur fracture surgery [7]. It can be argued hypoglycemia leads to the construction of more sensitive permanent regions in the brain even when the blood glucose level is normal. Movement disorders are more common in diabetic uremic patients, although they can also occur in individuals with non-diabetic uremic and metabolic acidosis. The basal ganglia produce cerebrovascular dysautoregulation and metabolic problems as a result of high levels of uremic toxins and metabolic acidosis, making them more vulnerable to harmful causes together with the underlying diabetic microangiopathy [8]. Our patient showed no signs of metabolic acidosis or uremia. Long-term and severe low blood glucose levels can be much more effective in the development of cerebrovascular dysautoregulation and metabolic problems. Ischemia can also be caused by impaired cerebrovascular autoregulation and metabolic problems caused by hypoglycemia.
Why hypoglycemia causes a unilateral lesion on imaging and a unilateral clinical appearance is still unclear. Not just anatomically, but also biochemically, in terms of neurotransmitters and other factors, there may be disparities between the hemispheres. This can lead various parts of the brain to react to the injury in different ways. Our patient had right-lateralized hemichorea-hemiballismus as a result of a lesion occurring in the left caudate nucleus head caused by hypoglycemia. It is also claimed that the left hemisphere was more vulnerable to the pathophysiological processes than the right hemisphere and the prognosis of Parkinson’s patients with clinical symptoms from the left hemisphere is significantly worse [9].
In terms of the anomalies found in DWI during hypoglycemic periods, the authors believe that an increase of the extracellular space caused by hypoglycemia and membrane damage caused by cytotoxic edema may explain the abnormalities [2]. Although it was claimed in previous publications that the movement disorder that occurs in hypoglycemia-induced movement disorders is not related to the duration and severity of hypoglycemia, in our case, lesions not seen in classical T1-weighted examinations in the presence of prolonged and deep hypoglycemia were observed on DWI. This implies that, contrary to common assumptions, hypoglycemia’s duration and intensity may be linked to the development of movement disorders.
In conclusion, hyperintensity on T1-weighted MRI, which is a classic finding in movement disorders due to hypoglycemia, may not be seen in long and deep episodes of hypoglycemia. In this scenario, physicians should pay attention to MRI scans on diffusion-weighted imaging, especially in patients with long and deep episodes of hypoglycemic attacks, to avoid missing cerebral ischemia, which is not a common finding.