Introduction
Paracetamol (acetaminophen) has clear analgesic and antipyretic activities, with limited peripheral anti-inflammatory properties when compared to other non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, diclofenac or indomethacin [1–3]. Both in pregnant women and their newborns, there is sufficient and robust evidence that paracetamol has opioid sparing effects for major pain syndromes, and is effective to treat minor to moderate pain syndromes, or to treat fever with a good short-term safety profile [1].
Paracetamol can be administered by different (enteral or parenteral) routes. As such, it is the drug most commonly used to treat mild to moderate pain or fever, including in pregnant women and during the postpartum period. Recent studies have shown that eight out of ten women take at least one prescription or over-the-counter (OTC) medicine during pregnancy, with paracetamol being the most commonly used OTC drug (65%) [4]. Oral paracetamol is used to treat mild to moderate pain and fever during pregnancy or labour, while intravenous (IV) paracetamol serves as an effective analgesic in the immediate post-operative period, e.g. after caesarean delivery [5].
Along the same line, effective analgesia is also a crucial and valid part of the care provided to (pre)term neonates. Paracetamol is one of the analgesics to attain this target, with data on drug utilization suggesting that paracetamol is somehow a ‘rising star’ to handle neonatal pain or fever [6–8]. To further illustrate this, paracetamol prescription has been quantified (14%) in the EUROPAIN prospective cohort (6680 neonates), and is more frequently prescribed compared to sedatives/hypnotics (12%) in the neonatal intensive care setting (NICU) [9]. Furthermore, paracetamol also became one of the drugs used to treat (symptomatic) patent ductus arteriosus in preterm neonates [10]. Also, the association between maternal paracetamol use and fetal ductus arteriosus constriction or closure has more recently been reported, although this risk is difficult to quantify and is likely very low [11, 12].
Related to this extensive use, paracetamol exposure during pregnancy is perceived to have a good safety/efficacy balance with the lowest risks and greatest benefits [4]. However, there are specific aspects related to pharmacodynamics (PD, intended effects, but also unwanted side effects) that warrant a focused interpretation on the available data, especially during pregnancy (fetal exposure), or when administered in early infancy in (pre)term newborns [1, 6]. While there are sufficient data on short-term safety, there are still concerns over long-term side-effects following perinatal exposure. These concerns relate to atopy, male fertility, and – most commonly reported – impaired neuro-cognitive and behavioural outcome. These findings are at present mainly based on epidemiological perinatal observations, combined with postulated mechanisms and supported by animal experimental observations [1]. However, their main limitation is that epidemiological studies describe associations, not necessarily causal links, and confounding by indication (there is a reason why paracetamol is prescribed) is the most obvious limitation.
Atopy related risks are speculated to be driven by non-selective inhibition by paracetamol on peripheral cyclo-oxygenase (COX) activity. This occurs besides its central nervous system related mechanisms, in a low prostaglandin, low inflammation setting. Due to maturational immunity (lymphocytes, TH1 vs. TH2), this may interfere with antigen tolerance development and may induce atopy [13, 14]. The mechanism that links perinatal paracetamol exposure to male fertility is claimed to be related to reduced testosterone production [15, 16]. Fetal paracetamol exposure in the second trimester of pregnancy has been associated with cryptorchidism or hypospadias, while first trimester paracetamol exposure has been associated with a shorter ano-genital distance in male infants [15, 16]. The currently reported neuro-cognitive and behavioural outcome risks suggested an exposure/effect association and were strongest for hyperactivity and attention deficits [1, 17]. Suggested mechanisms are related to cerebral inflammation, or to metabolites such as cannabinoids. Masarwa et al. recently reported a systematic review on the link between prenatal paracetamol exposure and the risk for ADHD, autism spectrum disorder (ASD) and hyperactivity, and suggested a pooled risk ratio of 1.34 (95% CI: 1.21–1.47), 1.19 (1.14–1.25) and 1.24 (1.04–1.43) respectively, but acknowledged that these observational data remain susceptible to several sources of bias [18, 19]. In these epidemiological studies, confounding by indication is a relevant issue, as e.g. there is a recent report on the link between maternal fever during pregnancy and attention deficit hyperactivity disorder (ADHD, odds ratio 1.31 and 2.64 for one or at least two fever episodes in the first trimester of pregnancy). Importantly, this risk was neither mitigated nor inflated by paracetamol exposure [20].
The European Medicine Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) recommendation very recently (March 2019) assessed the evidence and concluded that the clinical impact and relevance of these potential associations remains at present still uncertain. Pharmacovigilance Risk Assessment Committee therefore decided not to adapt the existing guidance, but to add some specific sentences on these associations in the summary of product characteristics (SmPC, the leaflet) in the relevant section on fertility, lactation and pregnancy (adapted wording is written in italics and underlined, copied verbatim from the SmPC). “A large amount of data on pregnant women indicate neither malformative, nor feto/neonatal toxicity. Epidemiological studies on neurodevelopment in children exposed to paracetamol in utero show inconclusive results. If clinically needed, paracetamol can be used during pregnancy however it should be used at the lowest effective dose for the shortest possible time and at the lowest possible frequency” [21].
These uncertainties and the extensive and increasing exposure to paracetamol in perinatal medicine serve as an obvious and urgent call to generate additional data on neuro-cognitive and behavioural outcomes in both human cohorts, as well as juvenile animal models to further explore aspects related to causality, mechanisms or the presence and extent of potential risks. This includes long-term neuro-cognitive and behavioural outcome data in clinical studies, or as part of ongoing studies and cohorts to generate data and create certainties on the existence and extent of any potential negative effects. This can be achieved by introducing a ‘pharmacovigilance’ concept and approach as part of long-term outcome studies. The paper of Juurjärvi et al. on neuro-cognitive outcome at the age of 2 years in a cohort (n = 48) of former preterm (23th–31th weeks of gestation) neonates initially recruited in a prospective study on prophylactic paracetamol prescription to induce ductus arteriosus closure is a recent example that such data can be generated as secondary outcome reports. In this study, the Griffiths test (IQ score) was similar between exposed and non-exposed cases [22]. Along the same line, albeit within an interventional study and comparing oral paracetamol versus ibuprofen to induce closure of a patent ductus arteriosus in preterm neonates (< 30 weeks of gestation), there was no difference in neuro-cognitive outcome between exposed and non-exposed cases at the age of 18–24 months [23].
Besides these pharmacovigilance studies, fetal or juvenile animal studies are another research line or approach to generate additional information on the benefit/risk balance of paracetamol exposure before (fetus) or after delivery (as (pre)term neonate or infant). At best, such studies should also include the indication to administer paracetamol to consider simultaneously both exposure and indication. In a chronic inflammatory pain and morphine juvenile rat model, it was concluded that persistent pain (formalin model) and morphine treatment in a neonatal rat affected the long-term behavioural outcome in adulthood. More relevantly, some of these outcomes were attenuated when both (opioid administration to treat the formalin-induced pain) were co-administered [24].
Based on our background in perinatal clinical pharmacology in humans, we conducted a structured search on the currently reported fetal or juvenile animal models following paracetamol exposure to provide a qualitative and quantitative overview on preclinical data.
Material and methods
A structured search was performed on December 26, 2019, using a broad search strategy [(paracetamol) AND (rat or rats or mouse or mice or murine or animal or monkey or macaque or nonhuman)) AND (neurodevelopmental or adhd or mental or social cognition or brain or behaviour or memory or neurotoxic or autis)] in PubMed to retrieve the available preclinical studies related to paracetamol exposure and the topic of interest (neuro-cognitive and behavioural outcome, perinatal exposure). Subsequent inclusion criteria for the final dataset of studies were any topics related to fetal or juvenile animal studies on neurotoxicity, neuro-cognition or behavioural outcome or any other indicator of relevance to the nervous system, based on title, abstract and full paper. The list of titles was screened twice to ensure full and accurate retrieval, and uncertainties were discussed (KA, JvdA). Once the list was established, a reference check and citation verification (on PubMed and on the journal website) were performed for the included studies.
In an attempt to assess the quality of the pre-clinical studies, the risk of bias was assessed with SYRCLE’s risk of bias tool for preclinical studies [25]. This was performed by KA, and uncertainties were discussed with JvdA until consensus was reached. Because we anticipated a heterogeneous pattern in species, interventions and outcome reported, a data extraction form was used to collect the data on study population (number, species, age, gender), intervention (paracetamol exposure, duration, indication), and outcome to provide a quantitative, descriptive overview.
Results
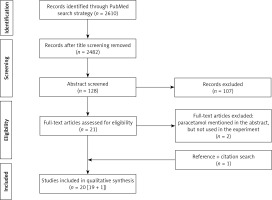
The above-mentioned search strategy resulted in 2610 hits in PubMed for screening. Based on title screening, 128 potential relevant hits were retained, to result in 21 potential hits after abstract assessment. Two additional papers were excluded after reading the full paper version (in both because paracetamol was not used in these studies, but mentioned in the abstract), and one additional paper was included following citation verification to result in 20 papers (Prisma flowchart, Figure 1).
Table I provides an overview on the quality assessment (SYRCLE’s risk of bias tool) results [26–45]. This reflects a heterogeneous pattern with mainly issues related to blinding (for performance, for detection) procedures in a relevant portion of the studies (unclear or unreported, high risk in 16/20 for both) and allocation concealment (was the method used to conceal the allocation sequence described in sufficient detail to determine whether intervention allocation could have been foreseen before or during enrolment?). Power calculations were not reported in any of the studies. This does not mean that these aspects were not considered during the design or study conduct, but we were not able to extract this information from the methods section description. Table II provides an overview on the characteristics of these studies [26–45]. Juvenile species reported are rat (n = 9), zebrafish larvae (n = 6), or mice/mouse (n = 5). Eight studies discussed embryological or fetal aspects of paracetamol exposure, 6 reported on juvenile animals (postnatal, but equivalent to (pre)term human age), while 6 studies combined pregnancy and juvenile exposure in the same animal. Within a given animal model, there is relevant variability in paracetamol dosing, while exposure has not been quantified. Paracetamol exposure in the zebrafish or rat models varied between the different research groups and studies.
Table I
| Study reference | Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding performance | Random outcome assessment | Blinding detection | Incomplete outcome data | Free from selective outcome bias | Additional comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Blecharz-Klin 2015 [26] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups? | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Male rat pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| Blecharz-Klin 2015 [27] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups? | ?, high risk Weight d60 differs | ?, high risk Weight d60 differs | Y, low risk | Y, low risk | Male rat pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| Blecharz-Klin 2016 [28] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups? | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Male rat pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| Blecharz-Klin 2017 [29] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Male rat pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| Blecharz-Klin 2018 [30] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Male rat pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| Blecharz-Klin 2019 [31] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk Co-housing within groups? | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Male pups, subtherapeutic chronic dosing; blinding and randomization procedure not fully described |
| David 2009 [32] | Y, low risk | ?, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, 0.1% ethanol co-group co-added, blinding and randomization not mentioned |
| Ellis 2018 [33] | Y, low risk | ?, low risk | ?, high risk | Y, low risk | ?, high risk Two larvae/well | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, nociception recovery model, paracetamol one of the known analgesics |
| Escapa 2019 [34] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, toxicity assessment effluent containing paracetamol |
| Hay-Schmidt 2017 [35] | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Mice, male brain (sexually dimorphic nucleus) and adult sexual behaviour following paracetamol vs. aniline vs. placebo during pregnancy |
| Klein 2020 [36] | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Rat, gestational exposure to paracetamol, both sexes, behaviour and neurochemical analysis |
| Leroux 2010 [37] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Excitotoxic brain (ibotenate intracerebral injection) injury model, effect of different NSAIDS, including paracetamol |
| Nogueira 2019 [38] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, heterogeneous pattern of outcome variables, ‘environmental’ concentrations |
| Philippot 2017 [39] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Mice, paracetamol exposure, critical age window and gender-related differences at adult age |
| Study reference | Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding performance | Random outcome assessment | Blinding detection | Incomplete outcome data | Free from selective outcome bias | Additional comments |
| Philippot 2018 [40] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Mice, paracetamol exposure, male, +/- cannabinoid agonist, behaviour and neurochemical at adult age. |
| Reuter 2016 [41] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, paracetamol sub-liver-toxicity dose in the latrophilin 3 ADHD and standard model. Hyperactivity as outcome |
| Saeedan 2018 [42] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, high risk Weight d60 differs | ?, high risk | Y, low risk | Y, low risk | Albino rat, paracetamol co-administration to a vaccine-induced fever model, behaviour and neurochemical at juvenile age |
| van den Hoogen 2016 [43] | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Rat, repetitive needle-prick model, paracetamol, short- and long-term hypersensitivity |
| Viberg 2014 [44] | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Y, low risk | Mice, male, adult behaviour and cognitive deficit after neonatal paracetamol exposure |
| Xia 2017 [45] | Y, low risk | Y, low risk | ?, high risk | Y, low risk | ?, high risk | ?, low risk | ?, high risk | Y, low risk | Y, low risk | Zebrafish larvae, ibuprofen vs. diclofenac vs. paracetamol, hatch and motor behaviour |
Table II
Study characteristics of the 20 individual studies retained in the search (alphabetically)
| Study reference | Animal | Fetal or pregnancy | Postnatal, juvenile | Intervention | Outcomes | Relevant findings |
|---|---|---|---|---|---|---|
| Blecharz-Klin 2015 [26] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60 (3 × 9 animals), only male rats | Spinal cord mono-amines, amino acids and blood chemistry (renal, liver, muscle safety) on day 60 | 3-methoxy-4-hydroxyphenylglycol (MHPG) and dopamine higher in controls, aspartate was higher in cases, reflecting modulation of dopaminergic and noradrenergic systems |
| Blecharz-Klin 2015 [27] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60 (3 × 10 animals), only male rats | Biochemical alterations in medulla oblongata + safety assessment on day 60 | Noradrenaline and dopamine, homovanillic acid, taurine, alanine higher, 5-hydroxy tryptamine (5-HT) lower in controls, reflecting modulation of serotonergic, dopaminergic and noradrenergic systems. Body weight on day 60 was different |
| Blecharz-Klin 2016 [28] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60 (3 × 10 animals), only male rats | Cerebellar levels of mono-amines and amino-acids | MHPG higher in cases, and 5-hydroxy-indole-acetic acid (5-HIAA) higher in the 5 mg cases, reflecting modulation of serotonergic and noradrenergic systems |
| Blecharz-Klin 2017 [29] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60, decapitation (3 × 10 animals), only male rats | Neurotransmitters (prefrontal cortex, hippocampus, striatum), spatial memory and motor performance | Dopamine higher in cases (striatum), 3-methoxytyramine (3-MT) higher (prefrontal); 5-HT higher and 5-HIAA lower (prefrontal) in 15 mg cases, reflecting modulation of serotonergic and dopaminergic systems. Staircase: no differences; hole board: motor activity lower in 5 mg cases; water maze: platform crossing higher in 5 mg cases; spatial reversal: elevated latency in 5 mg cases; reversal probe: swim distance and speed higher in 5 mg cases, related to difference in amino acid levels |
| Blecharz-Klin 2018 [30] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60 (3 × 10 animals), only male rats | Brain-derived neurotrophic factor (BDNF, prefrontal cortex, hippocampus, striatum), social behaviour and exploration | BDNF 2-fold lower (striatum) in exposed cases. Social interaction: higher frequency of social interaction and sniffing, but lower pinning in controls. Novel objection recognition: higher in 15 mg cases |
| Blecharz-Klin 2019 [31] | Rat | + | + | Placebo, 5 or 15 mg/kg/day paracetamol, from pregnancy to lactation until day 60 (3 × 10 animals), only male rats | Biochemical alterations in hypothalamus on day 60 | MHPG and glutamic acid higher in controls, and dopamine higher in 5 mg cases, reflecting modulation of dopaminergic and noradrenergic systems |
| Study reference | Animal | Fetal or pregnancy | Postnatal, juvenile | Intervention | Outcomes | Relevant findings |
| David 2009 [32] | Zebrafish | +, larvae | – | Developing eggs (30), exposed to different paracetamol (0, 1, 5,10, 50, 100 mg/l) levels for 7 consecutive days | Early development, hatching, organogenesis, larval growth and morphology, tail and tail-fin, pigmentation, behaviour and survival | Dose-related lower survival and affected embryo development; if successful, also delayed hatching; lower body mass; lack of pigmentation; malformations more present |
| Ellis 2018 [33] | Zebrafish | +, larvae | – | Nociception model (acetic acid recovery), post exposure normalization of activity (150 min) | Does paracetamol, ibuprofen or tramadol affect post exposure normalization | Paracetamol has no early, but a late effect (reduced hyperactivity after acetic acid exposure) |
| Escapa 2019 [34] | Zebrafish | +, larvae | – | Environmental toxicity assessment, zebrafish embryo assay (paracetamol in effluents) | Toxic effects (e.g. developmental delay, length, pigmentation, hatching) | Paracetamol displays concentration-related negative effects in the zebrafish embryo model |
| Hay-Schmidt 2017 [35] | Mice | + | – | Maternal paracetamol (50 or 150 mg/kg/day) or aniline (30 or 90 mg/kg/day), compared to placebo, gavage) from day 7 until delivery (5 × 10 pregnant animals) | Week 8 pups. Sexually dimorphic nucleus (sdn, preoptic area) anatomy; behavioural (urinary, aggression, sexual) testing (150 mg) | Paracetamol (150 mg) and aniline both decreased (50%) SDN cell number. Less masculine territorial marking, less territorial display, reduced mating |
| Klein 2020 [36] | Rat | + | – | Maternal paracetamol (350 mg/kg/day), from day 6 until delivery (gavage, 40/47 litters included) | Offspring behavioural testing (nest seeking, behavioural stereotypy, 3 chamber sociability, open-field, elevated plus-maze, hot plate); neurochemical (prefrontal cortex, hippocampus) | In paracetamol group, nest seeking: impaired; stereotypy: augmented apomorphine-induced; elevated plus-maze: decreased rostral grooming. Neurochemical assessment (glutathione, lipid hydroperoxide, bdnf): no differences |
| Leroux 2010 [37] | Mouse | – | + | Excitotoxic brain injury, ibotenate cortical injection. Aspirin, indomethacin, paracetamol (10, 100 µg) before/after injection | Lesion size on day 10 (pathology) | Post-treatment with paracetamol protected against white matter lesions, pre-treatment did not |
| Nogueira 2019 [38] | Zebrafish | +, larvae | – | Effects of environmental realistic exposure to paracetamol (0.005–3.125 mg/l) | Embryonic development, locomotor activity, biochemical, epigenetics | In paracetamol exposed: embryotoxicity (pigmentation, deformities); higher locomotor activity; increased cholinesterase, glutathione peroxidase, lipid peroxidation; methylation differs |
| Philippot 2017 [39] | Mice | – | + | Mice, male/female, exposed to paracetamol (2 × 30 mg/kg) or saline (subcutaneous) on postnatal day 3, 10 or 19 | Spontaneous behaviour (locomotion, rearing, total activity) in a new environment at adult equivalent age (2 months) | Adverse effects on behaviour and cognition on day 3 and 10, but not 19 in both male and female mice |
| Study reference | Animal | Fetal, or pregnancy | Postnatal, juvenile | Intervention | Outcomes | Relevant findings |
| Philippot 2018 [40] | Mice | – | + | Male mice, paracetamol (2 × 30 mg/kg) +/– cannabinoid receptor type 1 agonist (WIN 55 212-2) on day 10. Short-term: 24 h after initial exposure; long-term: adult mice | Short-term: transcript levels (cerebral cortex, hippocampus). Long-term: spontaneous behaviour (locomotion, rearing, total activity) in adult male mice (new environment) | In paracetamol + WIN co-exposed mice: short-term: fatty acid amide hydroxylase (cortex), synaptophysin and tropomyosin receptor kinase B (hippo) lower. Long-term: lack of habituation |
| Reuter 2016 [41] | Zebrafish | +, larvae | – | Zebrafish, exposed to sub-liver toxic levels of paracetamol (up to 1000 (acute), 100 (chronic) mg/l) | Locomotor activity levels, wild vs. latrophilin-3 (attention deficit hyperactivity disorder (ADHD) model) | No ADHD trait in larvae exposed to paracetamol |
| Saeedan 2018 [42] | Albino rat | – | + | Fever induced in pups (vaccines (MMR, DPT), or LPS) +/– paracetamol (subcutaneous, 50 mg/kg) | Growth; behaviour (swimming, olfactory, geotaxis, nociception, locomotor activity); pro- and anti-inflammatory markers | Paracetamol (+/–) exposed: weight gain differs; decreased locomotor activity; negative geotaxis; increases thermal nociception time; swimming behaviour, nest seeking, olfactory discrimination and exploration affected. Correlation between behaviour and pro- or anti-inflammatory markers |
| van den Hoogen 2016 [43] | Rat | – | + | Repetitive needle pricking model (day 0–7) +/– paracetamol (30 to 20 mg/kg, subcutaneous) vs. sham handling, n = 3 × 14 pups | Short- (mechanical hypersensitivity, von Frey) and long-term effects (postoperative hypersensitivity, ipsilateral paw incision 8th week) | No effects on short-term mechanical sensitivity or on baseline (weeks 3–8). Reduced duration of mechanical hypersensitivity after incision at adult equivalent age after neonatal exposure |
| Viberg 2014 [44] | Mice | – | + | 10-day-old mice, one or two paracetamol doses (30 mg/kg, subcutaneous) vs. normal saline. Only data in male mice collected | Short- (BDNF, frontal, parietal, hippocampus, day 11) and long-term behavioural effects (spontaneous behaviour, radial arm maze, hot plate, elevated plus maze) at 2 months | BDNF higher (frontal, parietal) in paracetamol cases. Altered locomotor behaviour in novel home cage, impaired spatial learning, but not thermal nociception or anxiety related behaviour |
| Xia 2017 [45] | Zebrafish | +, larvae | – | Ibuprofen, diclofenac or paracetamol (5–500 µg/l) effects on the zebrafish embryo | Short-term effects of ibuprofen, diclofenac or paracetamol on hatch, motor behaviour and RNA extraction | Paracetamol hatch: no effects; behaviour: no effects; RNA neuron related genes no effects |
The majority (n = 16) of the studies retained in the analysis were based on paracetamol exposure without indication for exposure, except for the induced fever model and the repetitive needle pricking model (both in rats), the excitotoxic brain injury model (mouse), and the zebrafish larvae nociception model.
Outcomes relate to biochemical alterations (mono-amines, amino acids, protein expression in the central nervous system), anatomical (teratogen, morphology, nuclear size) or behavioural (spatial memory, motor performance, social behaviour and exploration, sexual behaviour) aspects. Both within as well as between different animal models, the outcome was not always ‘uniform’. To illustrate this, BDNF is 2-fold lower (rat = striatum), similar (rat = prefrontal cortex, hippocampus; mice = hippocampus) or higher (mice = frontal and parietal).
Discussion
With this structured search, we aimed to provide an overview on the currently available animal models on neuro-cognitive and behavioural aspects following paracetamol exposure, with the a priori intention to reflect on their applicability for the human perinatal setting. This is because these animal experimental studies can be of relevance to explore aspects related to causal mechanisms or the presence and extent of potential risks for the perinatal human setting. In this way, these models may assist to close the gap between epidemiological association and causality.
Related to the causal mechanism, the cumulative data generated in juvenile animals provide support for an interesting ‘cannabinoid’ hypothesis to link paracetamol exposure and neuro-cognitive and behavioural impaired outcome. Pregnancy (maternal, fetal) and preterm specific aspects of paracetamol metabolism have been described [5, 46]. Paracetamol drug metabolism also includes an active metabolite (p-aminophenol) that interacts with cannabinoid receptors. Related to this, paracetamol and Δ(9)-tetrahydrocannabinol, but not ibuprofen, resulted in developmental neurotoxicity in a mouse model [39, 40, 47]. A single injection of Δ(9)-tetrahydrocannabinol on postnatal day 10 altered adult spontaneous behaviour and habituation rates in adult mice, similar to the pattern observed following acetaminophen exposure [40, 47, 48]. Furthermore, co-administration of a cannabinoid receptor agonist enhanced the developmental neurotoxicity of paracetamol in the same mouse model [40]. This suggests that the experimental animal and perinatal epidemiological research lines in humans on cannabinoids and paracetamol should further interact and liaise [49]. An alternative hypothesis relates to reduction of cerebral COX-activity as there is evidence that COX-2 polymorphisms (affecting phenotypic COX activity) explain in part the differences in cognitive outcome in former preterm neonates [50]. However, ibuprofen in the same mouse model did not result in the developmental neurotoxicity [47].
The overview provides a heterogeneous pattern of studies on neuro-cognitive and behavioural outcome following paracetamol administration in a limited number of small species, but without data in ‘higher animals’ such as the rabbit, or when very focussed, topical questions are raised, sheep or monkey. Neuropathological and -behavioural consequences of preterm birth have recently been described in the rabbit, and this model has very recently been applied to quantify the effects of caffeine exposure [51, 52]. For sheep, a chronically catheterized fetal sheep model to assess effects of non-narcotic analgesics has been described, while this species has been used to assess the impact of steroids on the developing brain [53, 54]. As a final illustration, the fetal neurotoxicity of dexmedetomidine has been studied in a pregnant cynomolgus monkey model, as well as a paracetamol hepatotoxicity model in the same species [55, 56].
Besides diversification in species, there are 3 other aspects that we feel – driven by our background in perinatal clinical pharmacology in humans – may be of relevance to further consider the benefit/risk balance. First, interspecies similarities in paracetamol dose/exposure should be further assessed. The variability in paracetamol dosing for the different animals in the absence of data on exposure makes it difficult to retrieve the signal or trend and link pharmacokinetics to pharmacodynamics (Table II). Second, we should further discriminate between acute (< 1 week human equivalent age) and chronic exposure. Related to this, animal models that collect data following chronic and ongoing exposure (like the Blecharz-Klin model) during sample collection have their scientific value. However, such a design makes it difficult to disentangle the effects related to the acute (ongoing) and chronic exposure.
Finally, the current models do not yet reflect the clinical setting as indications should be integrated in these models. It is quite obvious that non-exposure is the safest setting if there is no indication to administer paracetamol, but safety/efficacy assessment is a balanced exercise considering both indication (pain, fever, ductus arteriosus) and exposure. To further illustrate this, we refer to the Leroux, Saeedan and van den Hoogen models that assessed the impact of paracetamol integrated in a perinatal excitotoxic brain injury, a fever and a procedural pain model, respectively. Such models are closer to the earlier mentioned example of the effects of opioids in an inflammatory pain model [24]. There are some examples of such animal experimental design in the non-perinatal age window to assess neurological effects of paracetamol. Paracetamol reduces mitochondrial dysfunction during early cerebral post-ischemic reperfusion in rats [57]. Within the same topic, paracetamol potentiates endothelin-A receptor antagonist BQ123 induced hypothermia and reduces infarction following focal cerebral ischemia in rats [58]. Interestingly, and related to the cannabinoid hypothesis mentioned as a mechanism of toxicity, there is animal experimental evidence that cannabinoids are neuroprotective in perinatal asphyxia and newborn brain injury [59]. This suggests that it is worth the effort to explore the pharmacokinetics and -dynamics of paracetamol in perinatal asphyxia animal models and – when successful – in newborns undergoing whole body hypothermia. At least, this suggests that the toxicity of paracetamol should be explored beyond the well-known hepatotoxicity in children [60]. Along the same line, neuro-behavioural outcome should be included as a long-term outcome parameter of interest when paracetamol is prescribed to preterm neonates for e.g. patent ductus arteriosus during respiratory adaptation [10, 61].
Based on the structured search on perinatal animal models and neuro-cognitive and behavioural outcome following paracetamol exposure, we conclude that there is still limited species diversity with absence of ‘higher’ animal models. Furthermore, there is relevant within-species paracetamol dosing variability (dose, duration) with undocumented exposure. Based on our background in perinatal human clinical pharmacology, we highly recommend that these models should further integrate clinical indications, as non-exposure is the obvious safest setting in the absence of an indication. Besides pain and fever and related to the cannabinoid hypothesis, this should include perinatal brain injury, as there is animal experimental evidence that cannabinoids are neuroprotective in newborn brain injury or asphyxia, further supported by evidence in non-perinatal models of paracetamol-related neuroprotective effects.