Introduction
Subchorionic hematoma (SCH) refers to a collection of blood between the chorionic membrane and the uterine wall as caused by the separation of the chorion from the endometrium [1, 2]. It is the most common sonographic abnormality in pregnant women with symptoms of threatened miscarriage and the most common cause of first-trimester bleeding [3, 4]. The incidence of SCH is considered to range from 1.7% to 3.1% in the general obstetric population, whereas it may rise to 20% in pregnant women with symptoms of threatened abortion [3, 5].
Although SCH is a common abnormality suggested to be associated with adverse pregnancy outcomes such as early pregnancy loss, placental abruption and preterm delivery, the exact impact of SCH on pregnancy outcomes remains unclear with ongoing controversy regarding the risk of abortion in early pregnancies complicated by SCH as well as the clinical importance of the size of SCH in terms of adverse pregnancy outcomes [4, 6–13].
This study was therefore designed to investigate the association of SCH with adverse pregnancy outcomes in pregnant women in relation to size of hematoma and control pregnant women.
Material and methods
Study population
A total of 178 women with singleton pregnancy who had sonographically detected SCH in the first trimester (< 14 weeks) were included in this study conducted between January 2014 and January 2018 in a tertiary care hospital. A total of 350 pregnant women without SCH comprised the control group. The presence of fetal cardiac activity at the time of SCH diagnosis was a prerequisite for inclusion in the study, while presence of a nonviable fetus, multifetal pregnancy, fetal abnormality on ultrasonography and pre-gestational diabetes and/or hypertension comprised the exclusion criteria.
Written informed consent was taken from each participant. The study was approved by the Local Ethics Committee of Medeniyet University Göztepe Training and Research Hospital, Istanbul, Turkey.
Assessments
Data on maternal age, smoking status, gestational week at diagnosis, location of SCH, medications before diagnosis, gestational week at delivery, delivery route and pregnancy outcomes (first trimester vaginal bleeding, pre-eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), placental abruption, preterm delivery < 37 weeks, early pregnancy loss and intrauterine death) were retrieved retrospectively from hospital records.
Subchorionic hematoma diagnosis
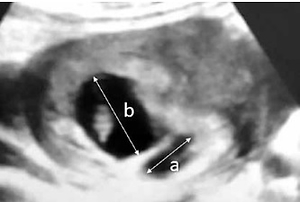
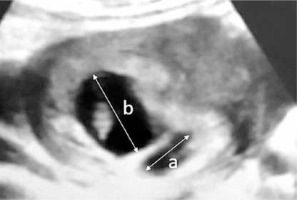
Subchorionic hematoma was diagnosed via transvaginal sonography (SonoScape S11, 2013) in the first trimester in all women by the same obstetrics and gynecology specialist. Pregnant women with SCH were divided into 3 groups according to the size of hematoma (the ratio of the largest linear diameter of SCH to the linear diameter of pregnancy sac) including small SCH (SCH-I group, ratio < 1/4, n = 47), medium-size SCH (SCH-II group, ratio 1/4-1/2, n = 110) and large SCH (SCH-III group, ratio > 1/2, n = 21) groups (Figure 1).
Pregnancy outcomes
Fetal loss was considered either early pregnancy loss (before the 22nd week) or intrauterine death (after 22nd week), depending on the pregnancy week. Pre-eclampsia was defined as new-onset hypertension (two consecutive measurements, at a 4-hour interval, of blood pressure > 140/90 mm Hg after the 20th week of pregnancy) and accompanying proteinuria (300 mg/day or +2 proteinuria with urine dipstick or spot urine protein/creatinine ≥ 0.3 mg protein/mg creatinine) [14]. Gestational diabetes was defined as diabetes diagnosed during the second half of pregnancy, based on a 50 g oral glucose tolerance test applied in the 24–28th weeks of pregnancy [15]. Intrauterine growth restriction was defined as fetal weight of < 10th percentile. Placental abruption was detected by the obstetrician at the time of delivery and placental examination. Delivery before the 37th week of the pregnancy was defined as preterm delivery.
Statistical analysis
Data obtained in the study were analyzed statistically using SPSS 25.0 software (IBM Corporation, Armonk, NY, USA). The conformity of the data to normal distribution was evaluated using the Shapiro-Wilk test, and homogeneity of variance was evaluated with the Levene test. Multiple independent groups of quantitative data (control group and SCH group) meeting parametric conditions according to age were compared using one-way ANOVA. When comparing according to diagnosis and gestational week at delivery, as non-parametric tests, the Kruskal-Wallis H test was used with Monte Carlo simulation technique results, and Dunn’s test was used for post hoc analyses. When comparing control and SCH groups (I, II and III) according to parity, gestational week at delivery, first trimester vaginal bleeding, cigarette smoking, route of delivery, early pregnancy loss, IUGR, placental detachment, preterm delivery, pre-eclampsia, gestational diabetes and intrauterine death, the linear-by-linear association and Fisher-Freeman- Holton tests were used with the Monte Carlo simulation method, and the column rates were compared with each other and expressed according to the Benjamini-Hochberg adjusted p-value results. Multiple ordinal logistic regression analysis was used to determine the cause-effect relationship between explanatory variables (such as gestational week at diagnosis, gestational week at delivery, first trimester vaginal bleeding, early pregnancy loss, IUGR, placental abruption, preterm delivery) and response variables (control and SCH groups). Quantitative variables were stated as mean ± standard deviation (SD) and median (minimum/maximum) values, and categorical variables as number (n) and percentage (%) in the tables. Variables were evaluated at a 95% confidence level and a value of p < 0.05 was considered as statistically significant.
Results
Althought not statistically significant, a tendency for association with earlier gestational age at diagnosis was noted in the larger size hematomas (median 8.0 weeks for SCH-III group, 8.3 weeks for SCH-II group and 8.9 weeks for SCH-I group). Subchorionic hematoma was associated with significantly lower gestational age at delivery (median (min–max) 38.29 (8.43–40.71), 37.93 (7.86–40.43) and 27.86 (7.86–37.57) weeks for SCH I, II and III groups, respectively vs. 38.86 (7.14–42.00) weeks in the control group, p < 0.001) and higher rate of first trimester bleeding (21.3%, 36.4% and 76.2% for SCH I, II and III groups, respectively vs. 6.0% in control group, p < 0.001) as compared with the control group, regardless of the size of the hematoma (Table I).
Table I
Comparison of pregnancy outcomes and clinical findings in control and subchorionic hematoma (SCH) groups
| Parameter | Control (n = 350) | SCH-I (n = 47) | SCH-II (n = 110) | SCH-III (n = 21) | P-value |
|---|---|---|---|---|---|
| Age [years], mean ± SD (min./max.) | 27.51 ±5.08 (18/39) | 27.04 ±5.31 (18/39) | 26.10 ±4.88 (18/39) | 27.62 ±5.13 (18/39) | 0.0941 |
| Hematoma localization, n (%): | |||||
| Anterior | – | 20 (42.6) | 53 (48.1) | 10 (47.7) | 0.8262 |
| Fundus | – | 8 (17.0) | 17 (15.5) | 3 (14.3) | |
| Posterior | – | 10 (21.3) | 15 (13.7) | 5 (23.8) | |
| Cervical | – | 9 (19.1) | 25 (22.7) | 3 (14.2) | |
| Prior medications, n (%): | |||||
| Progesterone | 32 (9.1) | 7 (14.9) | 14 (12.7) | 3 (14.2) | 0.6562 |
| LMWH | 16(4.6) | 2 (4.2) | 7 (6.4) | 1 (4.8) | |
| Aspirin | 6 (1.7) | 1 (2.1) | 2 (1.8) | 1 (4.8) | |
| None | 296 (84.6) | 37 (78.8) | 87 (79.1) | 16 (76.2) | |
| Gestational week at diagnosis, median (min./max.) | – | 8.9 (6.1/13.9) | 8.3 (6.6/13.3) | 8 (6.7/11.3) | 0.0943 |
| Gestational week at delivery, median (min./max.) | 38.86 (7.14/42.00)BC | 38.29 (8.43/40.71) | 37.93 (7.86/40.43) | 27.86 (7.86/37.57)AB | < 0.0013 |
| Gestational week at delivery, n (%): | |||||
| < 37 | 45 (12.9) | 8 (17.0) | 24 (21.8)A | 18 (85.7)ABC | < 0.0012 |
| > 37 | 305 (87.1)CD | 39 (83.0)D | 86 (78.2)D | 3 (14.3) | |
| Parity: | |||||
| Primipara | 171 (48.9) | 25 (53.2) | 59 (53.6) | 12 (57.1) | 0.2714 |
| Multipara | 179 (51.1) | 22 (46.8) | 51 (46.4) | 9 (42.9) | |
| 1st trimester vaginal bleeding: | |||||
| Absent | 329 (94.0)BCD | 37 (78.7)D | 70 (63.6)D | 5 (23.8) | < 0.0012 |
| Present | 21 (6.0) | 10 (21.3)A | 40 (36.4)A | 16 (76.2)ABC | |
| Cigarette smoking: | |||||
| Absent | 324 (92.6) | 44 (93.6) | 100 (90.9) | 19 (90.5) | 0.9172 |
| Present | 26 (7.4) | 3 (6.4) | 10 (9.1) | 2 (9.5) | |
| Route of delivery: | |||||
| CS | 68 (19.4) | 13 (27.7) | 25 (22.7) | 9 (42.9) | 0.0652 |
| Spontaneous | 282 (80.6) | 34 (72.3) | 85 (77.3) | 12 (57.1) | |
| Early pregnancy loss: | |||||
| Absent | 338 (96.6)CD | 43 (91.5)D | 96 (87.3)D | 12 (57.1) | < 0.0012 |
| Present | 12 (3.4) | 4 (8.5) | 14 (12.7)A | 9 (42.9)ABC | |
| IUGR: | |||||
| Absent | 339 (96.9)D | 46 (97.9)D | 104 (94.5)D | 16 (76.2) | 0.0032 |
| Present | 11 (3.1) | 1 (2.1) | 6 (5.5) | 5 (23.8)ABC | |
| Abruptio placenta: | |||||
| Absent | 347 (99.1)CD | 46 (97.9) | 105 (95.5) | 18 (85.7) | 0.0022 |
| Present | 3 (0.9) | 1 (2.1) | 5 (4.5)A | 3 (14.3)A | |
| Preterm delivery: | |||||
| Absent | 318 (90.9)D | 43 (91.5)D | 99 (90.0)D | 12 (57.1) | < 0.0012 |
| Present | 32 (9.1) | 4 (8.5) | 11 (10.0) | 9 (42.9)ABC | |
| Pre-eclampsia: | |||||
| Absent | 324 (92.6) | 43 (91.5) | 101 (91.8) | 19 (90.5) | 0.8922 |
| Present | 26 (7.4) | 4 (8.5) | 9 (8.2) | 2 (9.5) | |
| Gestational diabetes: | |||||
| Absent | 333 (95.1) | 45 (95.7) | 106 (96.4) | 20 (95.2) | 0.9582 |
| Present | 17 (4.9) | 2 (4.3) | 4 (3.6) | 1 (4.8) | |
| Intrauterine death: | |||||
| Absent | 348 (99.4) | 47 (100.0) | 109 (99.1) | 20 (95.2) | 0.2082 |
| Present | 2 (0.6) | 0 (0.0) | 1 (0.9) | 1 (4.8) |
Placental abruption (4.5% and 14.3% vs. 0.9%, respectively, p = 0.002) and early pregnancy loss (12.7% and 42.9% vs. 3.4%, respectively, p < 0.001) were significantly more common in SCH-II and III groups than in the control group (Table I).
The SCH III group had a significantly higher rate of < 37 gestational weeks at delivery (85.7% vs. 17.0% in SCH I, 21.8% in SCH II and 12.9% in control group, p < 0.001), first trimester vaginal bleeding (76.2% vs. 21.3% in SCH I, 36.4% in SCH-II and 6.0% in control groups, p < 0.001), early pregnancy loss (42.9% vs. 8.5% in SCH-I, 12.7% in SCH-II and 3.4% in control group, p < 0.001), IUGR (23.8% vs. 2.1% in SCH-I, 5.5% in SCH-II and 3.1% in control group, p = 0.003) and preterm delivery (42.9% vs. 8.5% in SCH-I, 10.0% in SCH-II and 9.1% in control group, p < 0.001) as compared with SCH-I and II groups as well as with control group (Table I).
No significant difference was noted between study groups in terms of age, SCH localization, medications before diagnosis, parity, cigarette smoking, route of delivery, gestational diabetes, pre-eclampsia and intrauterine death (Table I).
The risk for 1st trimester vaginal bleeding, early pregnancy loss, IUGR, and placental delivery increased with increase in size of SCH, while early pregnancy loss was the parameters associated with the largest increase in risk depending on the hematoma size (Table II).
Table II
Multiple ordinal logistic regression analysis showing pregnancy outcome risk in the SCH and control groups
Apart from higher likelihood of early pregnancy loss in anterior (15.7%) versus cervical (8.1%) and fundus (7.1%) hematomas (p = 0.046), no significant difference was noted in pregnancy outcomes with respect to hematoma localization (Table III).
Table III
Pregnancy outcomes with respect to hematoma localization
| Parameter | Anterior n (%) | Cervical n (%) | Fundus n (%) | Posterior n (%) | P-value |
|---|---|---|---|---|---|
| Early pregnancy loss: | |||||
| Absent | 70 (84.3) | 34 (91.9) | 26 (92.9) | 21 (70.0) | 0.046 |
| Present | 13 (15.7)a | 3 (8.1) | 2 (7.1) | 9 (30.0) | |
| IUGR: | |||||
| Absent | 77 (92.8) | 35 (94.6) | 25 (89.3) | 29 (96.7) | 0.737 |
| Present | 6 (7.2) | 2 (5.4) | 3 (10.7) | 1 (3.3) | |
| Placental abruption: | |||||
| Absent | 78 (94.0) | 35 (94.6) | 27 (96.4) | 29 (96.7) | 0.999 |
| Present | 5 (6.0) | 2 (5.4) | 1 (3.6) | 1 (3.3) | |
| Preterm delivery: | |||||
| Absent | 71 (85.5) | 31 (83.8) | 25 (89.3) | 27 (90.0) | 0.782 |
| Present | 12 (14.5) | 6 (16.2) | 3 (10.7) | 3 (10.0) | |
| Pre-eclampsia: | |||||
| Absent | 74 (89.2) | 35 (94.6) | 26 (92.9) | 28 (93.3) | 0.841 |
| Present | 9 (10.8) | 2 (5.4) | 2 (7.1) | 2 (6.7) | |
| Gestational diabetes: | |||||
| Absent | 80 (96.4) | 35 (94.6) | 27 (96.4) | 29 (96.7) | 0.941 |
| Present | 3 (3.6) | 2 (5.4) | 1 (3.6) | 1 (3.3) | |
| Intrauterine death: | |||||
| Absent | 82 (98.8) | 36 (97.3) | 28 (100.0) | 30 (100.0) | 0.787 |
| Present | 1 (1.2) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
Discussion
Our findings revealed an association of SCH regardless of the size of hematoma with lower gestational age at delivery and higher rate of first trimester bleeding in pregnant women, whereas increased likelihood of < 37 gestational week delivery, first trimester vaginal bleeding, early pregnancy loss, placental abruption, IUGR and preterm delivery was observed with increase in size of SCH.
The current study was based on subjective evaluation of hematoma size as a fraction of gestational sac size. This seems notable given that amongst the several methods of detecting the size of SCH reported in the literature this was the method considered to be best correlated with first trimester pregnancy outcome [16].
Our findings are consistent with the previously reported association of presence and/or size of SCH with adverse pregnancy outcomes such as increased likelihood of miscarriage [3–5, 10, 17, 18], IUGR [17], first trimester vaginal bleeding [2, 19], placental abruption [2–4], preterm delivery [2–4] and fetal growth restriction [3, 4].
In a meta-analysis of 7 studies in 1,735 women with SCH and 70,703 controls, the authors concluded that SCH was associated with an increased risk of spontaneous abortion (pooled OR = 2.18) and stillbirth (pooled OR = 2.09), abruption (OR = 5.71), preterm delivery (pooled OR = 1.40), and preterm premature rupture of membranes (pooled OR = 1.64), but not small for gestational age or pre-eclampsia [7].
In fact, the observation that the SCH-III group had the highest risk of early pregnancy loss in our cohort seems in agreement with data from a past study indicating a cut-off value of 32 ml for the size of hematoma, with a higher rate of miscarriage for hematomas larger than 32 ml with 81% sensitivity in ROC analysis [17]. Similarly, in a past study on prognosis of very large (> 50% of the gestational sac) first-trimester hematomas, the authors noted the association of very large hematomas with adverse outcome in almost half of the pregnancies along with worse outcomes when the hematoma was diagnosed at an early gestational age [20]. This seems also notable given that larger hematomas were associated with earlier gestational age at diagnosis in our cohort.
In addition, association of presence and size of SCH with an increased risk for preterm delivery but not for intrauterine death in the current study seems consistent with similar rates of intrauterine death reported in pregnant women with and without SCH [3].
Data from a recent study indicated a higher rate of SCH in women with risk factors for poor placentation such as multiparity, suggesting the possible association of SCH with poor placentation [21]. Advanced maternal age or increased parity was also found to be associated with SCH in another study [2]. However, similar to our findings, no significant association of maternal age or parity with presence or size of hematoma in pregnancy was also reported in the literature [17].
Presence of SCH was also reported to increase the risk of miscarriage in patients with vaginal bleeding and threatened abortion during the first 20 weeks of gestation, but with no significant impact on the outcome measures of ongoing pregnancies such as gestational week at delivery, birth weight, and delivery route [5]. In addition, in a meta-analysis of six studies, the authors confirmed the association of SCH with an increased risk of spontaneous abortion but not with a significant difference in premature delivery rates and delivery mode as compared with the threatened abortion group [10]. Notably, in our cohort, although the delivery route was similar in study groups, presence of hematoma was associated with delivery at an earlier gestational week compared to the control group, alongside an increased risk of earlier gestational age at delivery in pregnant women with larger vs. smaller hematomas. In addition to increased risk of preterm delivery, larger hematomas were also associated with increased risk for placental abruption and IUGR in our cohort. This seems also notable given the suggested association of SCH in pregnancy with higher likelihood of fetal distress, based on its association with increased risk of operative vaginal delivery (vacuum extraction) or cesarean delivery as well as with higher risk of placental abruption or abnormal placentation compared to pregnancies without SCH [3]. Accordingly, presence of SCH was also reported to be associated with adverse neonatal outcomes including increased risk of low gestational age at birth, low birth weight, low Apgar score at 1 and 5 min and higher likelihood of admission to the NICU compared to control pregnant women [4].
Indeed, unlike the current study findings, past studies also reported no association of preterm labor or spontaneous abortion with the size of SCH [3, 6] as well as an increased risk of pregnancy-induced hypertension and pre-eclampsia [2, 3, 21] or gestational diabetes [22] in the presence of SCH. It was also reported that that neither position nor location of the placental hematoma was associated with the pregnancy outcome [20], while a posteriorly located SCH has been associated more with fetal distress [3]. This seems notable given that localization of the SCH was similar with respect to size of the hematoma in our cohort.
In addition, while use of low dose aspirin [23], heparin [24] or thrombolytic therapy [25] has been suggested to be associated with an increased risk of developing a SCH during the first trimester pregnancy, our findings revealed no significant difference between SCH and control groups in terms of progesterone, heparin or aspirin usage rates. Lack of a significant impact of heparin or progesterone therapy on the risk or improvement of early pregnancy loss, respectively, has also been reported in other studies [23, 26, 27].
The exact pathophysiology of SCH-related adverse pregnancy outcomes has not been clearly elucidated, while premature perfusion of the intervillous space, mechanical effects of the hematoma, and impaired angiogenesis causing the hematoma have been suggested amongst the potential predisposing factors [5, 7, 21, 28, 29]. In this regard, albeit not analyzed in the current study, some authors emphasize the potential role of addressing gestational week at miscarriage or fetal distress (Apgar scores) parameters in better understanding of the pregnancy outcomes in women with SCH [3, 5].
Limitations and strength
The major strength of the current study seems to be assessment of hematoma size with a method best correlated with first trimester pregnancy outcome as well as inclusion of a large cohort of pregnant women with different sizes of SCH and a control group to analyze pregnancy outcomes. However, certain limitations to this study should be considered. First, due to the retrospective single center design of the present study, establishing the temporality between cause and effect is not possible. Second, the lack of data on SCH outcome (persistent or resolved), gestational week at miscarriage, or fetal distress (Apgar scores) is another limitation which otherwise would extend the knowledge achieved in the current study.
In conclusion, the findings of the current study indicate the increased likelihood of adverse pregnancy outcomes in first trimester diagnosis of SCH, including lower gestational age at delivery and higher rate of first trimester bleeding regardless of the hematoma size, but increased likelihood of < 37 gestational week delivery, first trimester vaginal bleeding, early pregnancy loss, placental abruption, IUGR and preterm delivery, particularly in those with larger size hematomas. Our findings emphasize the importance of serial follow-up of pregnant women with SCH via sonographic examination for the earlier detection of expected adverse pregnancy outcomes such as IUGR and to observe the clinical course of SCH. Further studies addressing the comparison of multiple methods of determining the effect of the size and location of SCH on pregnancy outcomes including the perinatal period might be useful in clinical settings.