Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
BIOLOGY MOLECULAR / RESEARCH PAPER
H2S treatment improves protective effect of mesenchymal stem cell-derived exosomes on renal ischemia/reperfusion fibrosis by suppressing inflammatory signaling of TGF-β and NF-κB as well as radical oxygen species generation
1
School of Nursing, Ningxia Medical University, Yinchuan, Ningxia, China
2
Department of Pathology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
3
Yinchuan Hospital of Traditional Chinese Medicine, Yinchuan, Ningxia, China
4
Ningxia Key Laboratory of Clinical and Pathogenic Microbiology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
5
The Third Department of Surgical Oncology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
Submission date: 2021-06-19
Final revision date: 2021-09-12
Acceptance date: 2021-09-26
Online publication date: 2021-10-29
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Hydrogen sulfide (H2S) has been reported to regulate signaling pathways responsible for development of renal ischemia/reperfusion (I/R) associated fibrosis. In this study, we hypothesized that preconditioning with H2S may boost the protective effect of mesenchymal stem cells- (MSC) -derived exosomes (EXOs) on renal I/R fibrosis.
Material and methods:
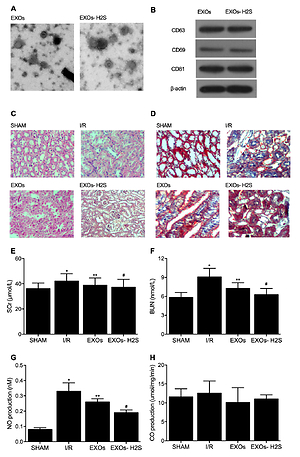
Real-time polymerase chain reaction (PCR) and Western blot were performed to evaluate mRNA and protein expression of Nrf2, NF-κB, TGF-β, α-SMA, and collagen type II (Col2). H & E and Masson staining were carried out to examine the kidney injury and fibrosis in rats.
Results:
H2S-preconditioned EXOs substantially promoted the protective effect of EXOs on the development of kidney injury as well as associated fibrosis in I/R rats.EXO treatment significantly restored the activated gene and protein expression of NF-κB, TGF-β, α-SMA, and Col2 in I/R rats and the cellular model of hypoxia/reoxygenation (H/R), while H2S preconditioning remarkably strengthened this effect of EXOs. Additionally, H2S preconditioning remarkably strengthened the efficiency of EXOs in restoring the activated expression of IL-1α, IL-6, IL-12 and TNF-β as well as altered activities of superoxide dismutase (SOD), malondialdehyde (MDA), H2O2, glutathione S-transferase (GST) and glutathione peroxidase (GPx) in I/R rats and the cellular model of H/R.
Conclusions:
This study utilized I/R rats to demonstrate that the effect of H2S-preconditioned exosomes in suppressing inflammation and radical oxygen species (ROS) generation was better than that of unconditioned exosomes. To be specific, exosomes, especially H2S-preconditioned exosomes, could not only reduce the expression of NF-κB and the downstream inflammatory responses, but also promote the expression of Nrf2 and inhibit ROS generation, which explained the molecular mechanism underlying the protective effect of H2S-preconditioned exosomes on renal I/R associated fibrosis.
Hydrogen sulfide (H2S) has been reported to regulate signaling pathways responsible for development of renal ischemia/reperfusion (I/R) associated fibrosis. In this study, we hypothesized that preconditioning with H2S may boost the protective effect of mesenchymal stem cells- (MSC) -derived exosomes (EXOs) on renal I/R fibrosis.
Material and methods:
Real-time polymerase chain reaction (PCR) and Western blot were performed to evaluate mRNA and protein expression of Nrf2, NF-κB, TGF-β, α-SMA, and collagen type II (Col2). H & E and Masson staining were carried out to examine the kidney injury and fibrosis in rats.
Results:
H2S-preconditioned EXOs substantially promoted the protective effect of EXOs on the development of kidney injury as well as associated fibrosis in I/R rats.EXO treatment significantly restored the activated gene and protein expression of NF-κB, TGF-β, α-SMA, and Col2 in I/R rats and the cellular model of hypoxia/reoxygenation (H/R), while H2S preconditioning remarkably strengthened this effect of EXOs. Additionally, H2S preconditioning remarkably strengthened the efficiency of EXOs in restoring the activated expression of IL-1α, IL-6, IL-12 and TNF-β as well as altered activities of superoxide dismutase (SOD), malondialdehyde (MDA), H2O2, glutathione S-transferase (GST) and glutathione peroxidase (GPx) in I/R rats and the cellular model of H/R.
Conclusions:
This study utilized I/R rats to demonstrate that the effect of H2S-preconditioned exosomes in suppressing inflammation and radical oxygen species (ROS) generation was better than that of unconditioned exosomes. To be specific, exosomes, especially H2S-preconditioned exosomes, could not only reduce the expression of NF-κB and the downstream inflammatory responses, but also promote the expression of Nrf2 and inhibit ROS generation, which explained the molecular mechanism underlying the protective effect of H2S-preconditioned exosomes on renal I/R associated fibrosis.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.