Introduction
Aortoiliac occlusive disease triggered by atherosclerosis is a serious cause of mortality and morbidity worldwide. It begins at the terminal aorta and proximal common iliac artery, and slowly progresses distally and proximally, in time, leading to a serious stenosis or total aortoiliac occlusion [1]. In this case, aortofemoral bypass (AFB, bilateral or unilateral) is still the golden standard treatment option despite the advancements in endovascular techniques [2, 3]. It is a commonly known fact that oxidative stress is a significant cause for atherosclerosis [4].
Abdominal aortic aneurysms (AAAs) are permanent localised dilatations in which the diameter of the abdominal aorta is larger than 3 cm. It is a complex and multifactorial disease with a strong genetic component [5]. Xiong et al. revealed that the initial changes in an experimental model of aneurysms were caused by the local production of reactive oxygen species (ROS) [6]. Comparison of endovascular AAA repair (EVAR) with open repair showed EVAR procedures offering excellent early and in terms of safety and reduction of mortality [7]. Affecting gene expression via DNA methylation, smoking is the most important environmental factor in the development, progression, and rupture of the disease [8].
Oxidative stress arises from an imbalance between production of ROS and antioxidant capacity of the biological system. Manganese superoxide dismutase (MnSOD), glutathione peroxidase (GPx), and catalase (CAT) enzymes all have critical importance in the antioxidant system [8]. Significance of antioxidant stress is highlighted in the pathogenesis of numerous diseases. Studies have also been conducted to determine the gene polymorphisms of these enzymes [9–13]. However, no genetic study on these antioxidant enzyme gene polymorphisms in the patient group investigated in the present study was found in the literature review.
Smoking is also a cause of oxidative stress. Epigenetic modification of chromosomes is changed by oxidative stress and smoking. Histone acetylation and inflammatory gene promoter methylation increase the over-expression of inflammatory genes in the epithelial cells [14].
The aim of this study was to determine the genetic polymorphisms of these antioxidant enzymes together with oxidative stress and the response of some antioxidant enzymes against this situation in vascular and endovascular interventions performed for smoking related diseases of the infrarenal abdominal aorta. MDA blood levels, as an indicator of oxidative stress, reduced glutathione (GSH), CAT, and SOD, which are indicators of antioxidant status. Blood levels were measured in AFB in AODs, and in EVARs in the preoperative, operative, and postoperative periods. Genetic polymorphisms of these antioxidant enzymes developing a response to the damage in the preoperative blood samples were determined by using the PCR-RFLP method.
Material and methods
Preparation of the patients for the operation and sample collection
All the patients (8 patients) suffering from aortoiliac occlusion and/or stenosis and AAAs (16 patients) were hospitalised at our centre one or 2 days earlier, in order to make their surgical preparations. These two groups were evaluated together because both of these diseases of the abdominal aorta are related with smoking and all patients were smokers or ex-smokers. Also, this was a single-centre pilot study, and so the patient populations were small. Informed written consent was obtained from all patients. Ethics Committee approval was obtained for the study from Fırat University in our city (2015-15).
Once endovascular grafts were prepared in appropriate sizes for 16 patients with AAAs according to their computed tomography (CT) angiography, they were brought to our centre (Medtronic Endurant II). Then, radial artery catheters were inserted to obtain preoperative samples and to allow appropriate monitoring together with the first blood gas analysis. Each of these patients was positioned on the operating table, and spinal anaesthesia was given through drugs containing heavy marcain and fentanyl. Patients were then taken to the angiography laboratory. At the angiography laboratory, the anaesthesia device and all kinds of infusion pumps were prepared. Invasive blood pressure, ECG, and PO2 monitoring were carried out. During the procedure, the patient was sedated with midazolam when required. EVAR was applied from the common femoral arteries by opening both groins. At the end of the procedure, all the patients were taken to their beds in the intensive care unit without mechanical ventilation. In the meantime, operative samples were taken from the radial artery catheter. Almost 24 h after the procedure, postoperative samples were taken in the same way from the patients.
Eight patients having AOD were taken to the operating room on the morning, and after appropriate monitoring they were intubated and general anaesthesia was induced by administering 100 mg lidocaine IV, 300 mg magnesium IV, 100 mg fentanyl IV, 0.6–1.2 mg/kg Esmeron IV, and 2 mg/kg propofol IV. The anaesthesia was maintained performed with isoflurane inhalation anaesthesia, fentanyl and Esmeron. Preoperative samples were taken from the radial artery catheter inserted before anaesthesia. Midline abdominal incision, retroperitoneal approach, and infrarenal cross clamping as well as AFB using Dacron prosthetic grafts were performed. In the postoperative period, the patients were taken to the intensive care unit with mechanical ventilation. Here, the operative samples were taken from the radial artery catheter. A third set of postoperative samples were taken from the patients about 24 h later. No mortality occurred in either patient group.
Sample collection
Once blood samples were taken in two heparin-containing experimental tubes, they were taken to the laboratory. While one of the heparinised bloods was used as the full blood, the other heparinised blood was centrifuged at 3000 rpm for 5 min. Its plasma was separated and washed three times by using physiological saline solution. Then, it was kept in the deep-freezer at –80ºC before biochemical analyses. MDA, SOD, GSH, and CAT measurements were done by Mrs. Doğan by manual method in the laboratories of Fırat University, Faculty of Veterinary, Department of Biochemistry. Genetic analysis was done by Mr. Özşensoy in Sivas Cumhuriyet University, Faculty of Veterinary Medicine, Department of Veterinary Biometrics and Genetics.
Biochemical analyses
Lipid peroxidation
The MDA assay in plasma was conducted with slight modifications according to the method of Placer et al. The resulting MDA created a pink complex with thiobarbituric acid (TBA), and the absorbance was read at 532 nm. The plasma MDA content was expressed as nmol/ml [15].
Reduce glutathione level
The educe glutathione (GSH) contents were determined according to the method of Chavan et al. and were expressed as μmol/g Hb [16].
Catalase activity
The Aebi method was used for the measurement of CAT activity.
The degradation rate of H2O2 by CAT was spectrophotometrically measured based on the fact that H2O2 absorbs light at a wavelength of 240 nm. CAT activity was calculated as katal/g Hb [17].
Superoxide dismutase activity
The SOD enzyme activity was measured based on nitro blue tetrazolium (NBT) degradation by superoxide radical, which was produced with the xanthine-xanthine oxidase system. The formozan obtained at the end of the reactions exhibits a blue colour and maximally absorbs at 560 nm [18]. The SOD enzyme activity was calculated as U/g Hb.
Genetic analysis
Blood samples of 3 ml were taken in tubes containing K3EDTA and were brought to the laboratory under cold chain as soon as possible. The blood samples were kept at –20°C until the time of analysis. DNA isolation from the blood samples was extracted by using the standard phenol/chloroform method [19].
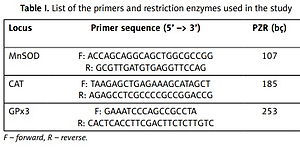
The DNA samples extracted were amplified to the gene region by using specific primers (Table I). PCR was performed in 15 μl volume including 1 × Mg++ free PCR buffer (Fermentas), 200 mM dNTPs (Fermentas), 1.5 mM MgCl++ (Fermentas), 0.375 U of Tag polymerase (Fermentas), 5 pMol each primer, and ~50 to 100 ng of genomic DNA in each reaction (Table I).
Table I
List of the primers and restriction enzymes used in the study
All the PCR products were amplified by using the Touchdown PCR profile [20]. The first step was initial denaturation at 95°C for 4 min, followed by 16 cycles of denaturation at 94°C for 30 s, with annealing beginning at 60°C and ending at 52°C for 30 s and elongation at 72°C for 30 s. The annealing temperature was decreased by 0.5°C per cycle until it reached 52°C. At the second step, 25 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 30 s was applied. The final elongation of 72°C for 10 min was applied in all reactions.
The PCR products obtained were digested by using the restriction enzymes specified in Table I. For the digestion process, a total of 30 ml was prepared as 10 ml PCR product, 1 ml restriction enzyme (fastdigest, 10 U/ml), 2 ml enzyme buffer, and 17 ml bidistilled water, and the samples were kept at 37°C for 20 min. The products obtained as a result of the enzyme restriction process were degraded in 3% agarose gel electrophoresis at 100 V for 45 min and viewed at 365 nm UV.
According to the genotypes to be obtained as a result of enzyme restriction, the lack of restriction in MnSOD gene (107 bç) gives the VV genotype, restriction of any of the allele genes (107, 89, 18 bç) gives the VA genotype, and restriction of both alleles (89, 18 bç) gives the AA genotype. The absence of restriction in CAT gene (185 bç) gives the TT genotype, restriction of any of the allele genes (185, 155, 30 bç) gives the TC genotype, and digestion of both alleles (155, 30 bç) gives the CC genotype. The absence of restriction in GPx3 gene (260 bç) gives the CC genotype, digestion any of the allele genes (260, 224, 36 bç) gives the TC genotype, and restriction of both alleles (224, 36 bç) gives the TT genotype.
Statistical analysis
While independent sample t-test was used for the comparison of the groups with the descriptive statistical data of all the biochemical data, Mann-Whitney U test was used for comparison of the routine biochemical data and the antioxidant parameters and oxidative damage of the patients undergoing EVAR and ABF. Statistical results were calculated by using SPSS v.15 software. Genotypic structures and allele frequencies of the study samples were determined by gene counting. Polymorphism differences were calculated by χ2 test.
Results
Although the mean MDA values between the groups in terms of preoperative, operative, and postoperative periods slightly increased, no statistically significant difference was determined (p > 0.05). There was a significant increase in GSH level in the operative and postoperative periods compared to preoperative period, but there was a significant increase in CAT activity in the postoperative period compared to the operative period (p < 0.05).
A significant decrease (p < 0.05) was observed in SOD enzyme activity in the postoperative period compared to the preoperative and operative periods (Table II).
Table II
Results of the oxidative and antioxidant parameters in patients with diseases of abdominal aorta in preoperative, operative, and postoperative periods
| Parameter | Preoperative period | Operative period | Postoperative period | P-value |
|---|---|---|---|---|
| MDA [nmol/ml] | 2.57 ±0.19a | 2.59 ±0.21a | 2.64 ±0.22a | > 0.05 (0.63) |
| GSH [μmol/g Hb] | 1.63 ±0.23a | 2.15 ±0.35b | 2.24 ±0.31b | < 0.05 (0.02) |
| CAT [k/g Hb] | 28.49 ±4.35ab | 27.37 ±3.38a | 30.58 ±3.47b | < 0.05 (0.00) |
| SOD [U/g Hb] | 33.06 ±1.92a | 32.79 ±1.43a | 31.22 ±1.24b | < 0.05 (0.01) |
A significant decrease was determined in MDA level in the postoperative period in the AFB group compared to the EVAR group (p < 0.05). GSH levels significantly increased in the preoperative period in the AFB group compared to the EVAR group (p < 0.05); no significant difference was determined in CAT activity (p > 0.05). A significant decrease was observed in SOD enzyme activity in the preoperative period in the AFB group compared to the EVAR group (p < 0.05) (Table III).
Table III
Statistical comparison of the oxidative and antioxidant parameters of patients undergoing EVAR and AFB operation in preoperative, operative, and postoperative periods
| Parameter | Preoperative period | Operative period | Postoperative period |
|---|---|---|---|
| MDA (EVAR) [nmol/ml] | 2.24 | 2.32 | 2.23a |
| MDA (AFB) [nmol/ml] | 2.07 | 2.2 | 1.88b |
| P-value | > 0.05 (0.13) | > 0.05 (0.27) | < 0.05 (0.02) |
| GSH (EVAR) [μmol/g Hb] | 0.97a | 1.72 | 1.02 |
| GSH (AFB) [μmol/g Hb] | 2.56b | 2.14 | 2.93 |
| P-value | < 0.05 (0.00) | > 0.05 (0.14) | > 0.05 (0.19) |
| CAT (EVAR) [k/g Hb] | 21.71 | 32.05 | 25.47 |
| CAT (AFB) [k/g Hb] | 15.66 | 14.88 | 17.94 |
| P-value | > 0.05 (0.07) | > 0.05 (0.06) | > 0.05 (0.06) |
| SOD (EVAR) [U/g Hb] | 31.17a | 35.53 | 31.15 |
| SOD (AFB) [U/g Hb] | 29.18b | 29.09 | 29.63 |
| P-value | < 0.05 (0.02) | > 0.05 (0.07) | > 0.05 (0.06) |
A statistically significant difference in laboratory measurements was found between the patients but they were not clinically significant (Table IV).
Table IV
Demographic data of patients with diseases of abdominal aorta
| Σn 24 | Groups | |||||
|---|---|---|---|---|---|---|
| Urea | Creatinine | AST | ALT | CRP | ||
| Presence of DM | Yes | 27.00 ±0.00a | 0.90 ±0.00a | 17.00 ±0.00a | 21.00 ±0.00a | 12.90 ±0.00a |
| No | 39.91 ±2.20b | 0.98 ±0.04b | 30.18 ±4.87a | 27.45 ±3.70a | 8.06 ±0.69b | |
| P-value | < 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 | |
| Presence of HT | Yes | 40.00 ±2.48a | 0.96 ±0.05a | 19.00 ±1.10a | 20.56 ±2.62a | 8.76 ±0.74a |
| No | 35.33 ±4.35b | 0.98 ±0.06a | 59.33 ±10.98b | 46.00 ±6.82b | 7.56 ±1.75b | |
| P-value | < 0.05 | > 0.05 | < 0.05 | < 0.05 | < 0.05 | |
| Smoking status | Smoking | 35.88 ±2.53a | 0.94 ±0.05a | 35.75 ±6.16a | 33.13 ±4.34a | 8.32 ±0.97a |
| No smoking | 44.75 ±3.25b | 1.02 ±0.04b | 15.75 ±0.97b | 14.50 ±0.86b | 8.75 ±0.82a | |
| P-value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | > 0.05 | |
| Presence of COPD | Yes | 42.00 ±3.11a | 1.06 ±0.06a | 31.83 ±7.67a | 29.00 ±5.09a | 8.19 ±1.31a |
| No | 35.67 ±2.79a | 0.88 ±0.04b | 26.33 ±5.04a | 24.83 ±4.67a | 8.73 ±0.54a | |
| P-value | > 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | |
According to the PCR and enzyme restriction results, the polymorphisms obtained as a result of the enzyme restriction of MnSOD, CAT, and GPx3 regions by using a total of 12 samples were determined. According to the polymorphism, the observed and expected allele genotypes and frequencies were determined. In the gel images, genotypes were distinguished according to presence or absence of 107 and 89 bp in MnSOD, 185 and 155 bp in CAT, and 260 and 224 bp in GPx3.
In MnSOD gene region two genotypes, VV (75%) and VA (25%), and two alleles, V (88%) and A (13%), were determined. Likewise, in the GPx3 gene region, two genotypes, TC (33.33%) and TT (66.67%), and two alleles, T (83%) and C (17%), were determined. In the CAT gene region, two genotypes, TC (66.67%) and CC (33.33%), and two alleles, T (33%) and C (67%), were determined. There was no statistical difference in terms of genotype obtained in the MnSOD, CAT, and GPx-3 gene regions.
Another result shows that although the increase of local ROS is one of the factors that are responsible for in the formation of both AAAs and AODs, lack of serious mutation or diversity in genetic polymorphisms of some of the antioxidant enzymes evaluated in this patient group indicated that different factors were at play in the development of diseases.
Discussion
Physicians performing vascular and endovascular interventions for infrarenal vascular disease have to treat an older and higher risk patient population. It is important to understand the effect of oxidative damage in determining the possible risks and mortality associated with interventions to improve management during and after the operation.
In the present study, MDA levels, an indicator of oxidative stress, showed a modest increase in operative and postoperative levels, but this increase was not statistically significant. This might be associated primarily with the increase in GSH and CAT levels, which were the parameters of the study, in preoperative and postoperative periods and furthermore such interventions did not result significantly in oxidative stress. When the open surgery and EVAR were compared, it was observed that MDA levels were almost the same in endovascular repair. The open surgery group undergoing AFB (bilateral or unilateral) had a significant decrease only in the postoperative period compared to the endovascular group. When the GSH levels were examined, it was observed that mean values were significantly higher in the AFB group than the endovascular group and there was a difference in favour of the significant AOD group rather than the preoperative period. A matter that should be considered here is that open surgery was carried out in descending aortoiliac occlusive disease with continuous ischaemia and reperfusion attacks. High levels of preoperative GSH and low levels of postoperative MDA may be related to these chronic ischaemia and reperfusion attacks.
In the study by Lucas et al. in 2016, aorta specimens received from 10 cadaver organ donors in the control group were compared with the specimen taken from infrarenal aorta of nine severe aortoiliac stenosis (SAS) and seven total aortoiliac occlusion (TAO) patients during open surgery. Although the aortic ROS levels were higher in the TAO group than in the SAS group (48.3 ±10.22 vs. 33.02 ±4.54 pmol/mg protein), both were significantly higher than the control group. Likewise, when the SOD activities were examined, it was stated that although there was no difference in TAO and SAS groups, both were higher than the control group. Also, CAT activity was in parallel with the SOD activity [21]. Of course, the fact that this study was built on local oxidant and anti-oxidant parameters and the cadaver control group were the most important factors to be criticised. Also, systemic oxidative status is highly valuable, but it can be affected by many systemic conditions like age and DM [22]. However, the increase in the local antioxidant enzyme activity showed itself at various points in this study. When EVAR and AFB groups were evaluated both individually and jointly, no difference was observed apart from the fact that MDA, a lipid peroxidation product, was statistically significantly lower in the AFB group only in postoperative period. Even in this case, mean values were very close to each other. Although both these diseases and surgical practices cause an increase in oxidative stress, it can be seen that antioxidant activity can balance this.
In the study conducted by Papalambros et al., in 2007 regarding the MDA results obtained from 53 AAA patients undergoing open surgery, baseline MDA levels were found to be significantly higher than in the control group. A close correlation was observed between aortic clamping periods and MDA levels in blood samples taken at the 15th and 60th min after reperfusion following intensive care transfer, and elimination of this difference should be associated with the possible changes in the levels of antioxidant enzymes at the 24th h, but these values were not measured in this study. As in the present study, the constant elevation of MDA levels after 24 h might be due to the increasing GSH and CAT activity [23].
There is a mutation region regarding the conversion of valine amino acid (Val, GTT) on the 47th nucleotide in the 9th position of the MnSOD gene, as well as the alanine amino acid [9]. Also, generally in studies it is reported that patients possessing homozygous AA genotype in the diseases are associated with disease risk [9]. In this study, while there was no patient having an AA genotype, mutations with A allele were seen at the rate of 13%.
It was found that there was a genetic variation in which A in the 861st nucleotide of the GPx3 gene was converted into T, and this mutation was associated with the disease risks [24]. In this study, T allele was seen at the rate of 83%.
In a previous study conducted on CAT gene, it was reported that T allele was significantly higher than the C allele; whereas, in the present study, no significance difference was observed between the alleles even though the C allele was present at the rate of 67% [10].
As a result, in the present study, the first findings regarding the antioxidant regions of patients included in a Turkish-origin sample group were obtained. The limitations of this study are the small patient groups and lack of comparison of the EVAR group with open surgery. Studies including further patients should be conducted to clearly determine which allele is associated with this disease.
In conclusion, in this single-centred pilot study, oxidative damage and some antioxidant parameters and genetic polymorphism of the antioxidant enzymes were discussed in endovascular repairs made for abdominal aortic aneurysms and aortofemoral by-passes in the aortoiliac lesions from daily routine procedures. From both perspectives, it is thought that the problems that may arise during these procedures, which are considered as very safe, are not related to oxidative damage.