Generalized arterial calcification of infancy (GACI) was initially identified by Bryant and White in 1901. The incidence is unknown, but around 1000 cases have been documented, mostly diagnosed post-mortem [1]. However, GACI has also been detected antenatally through fetal echocardiography [2]. Hajdu et al. [3] observed hyperechogenic and hypokinetic walls in both atria and ventricles, along with enlarged great vessels (the fetus died in utero). Samon et al. [4] diagnosed GACI antenatally by noting hyperechogenicity in the proximal aorta and central pulmonary vessels, with hypertrophic cardiomyopathy. In two other fetuses with GACI, the cardiac chambers were dilated, and severe myocardial dysfunction with atrioventricular valve regurgitation was evident. Additionally, hyperechogenic foci were found in the wall of both atria and great arteries. Dystrophic calcification in GACI leads to reduced vessel compliance and can cause systemic and/or pulmonary hypertension, resulting in ventricular hypertrophy or dilation, potentially leading to cardiac failure [5, 6]. Furthermore, GACI can cause fetal heart failure with polyhydramnios, fetal hydrops, and, ultimately, fetal demise during the early part of the last trimester of pregnancy [7, 8]. Postnatally, GACI presents with severe congestive heart failure and refractory systemic hypertension [9–11]. Medical treatment of cardiovascular effects is generally unsuccessful.
Therapeutic approaches
Relative to pharmacological interventions, some infants treated with bisphosphonate (an inhibitor of calcium salt precipitation) [12, 13] or a combination of corticosteroids, estrogens, and thyroid hormones have survived. Additionally, spontaneous remission of the disorder has been reported [14]. Etidronate, one of the first-generation bisphosphonates, has a profile favoring anti-mineralization over anti-osteoclastic activity. It has been shown to prevent ectopic mineralization in soft connective tissues [15]. At the same time, etidronate increases bone mineral density and corrects the mineralization defect noted in GACI, at least in animal models [15]. In patients with pseudoxanthoma elasticum, etidronate reduces arterial calcification and subretinal neovascularization events [16]. Therefore, etidronate may offer dual benefits by preventing ectopic mineralization and reducing bone loss over the long term through its anti-osteoclastic effect [17]. In adult patients with arterial calcification, etidronate can slow the progression of vascular disease and is both safe and well-tolerated [18].
GACI in twin-twin transfusion syndrome
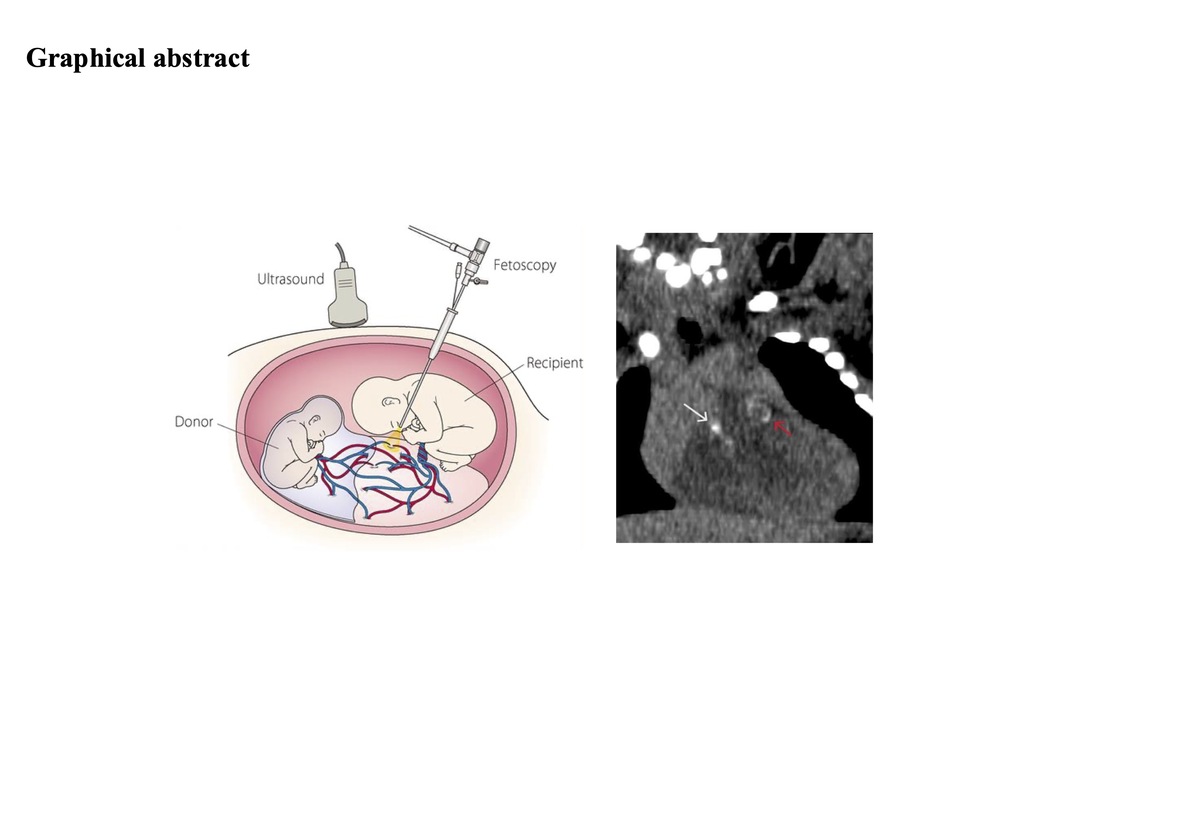
Concerning the topic of our study, GACI has been reported in two sets of monochorionic and dichorionic twins [19–21]. In these cases, both twins were affected and tested positive for ENPP1 (ecto-nucleotide pyrophosphatase/phosphodiesterase). One case of GACI was also described in the recipient twin of a twin-twin transfusion syndrome (TTTS) [4]. This latter is a condition where blood flow is uneven between twins sharing a placenta (monochorionic twins). TTTS affects about 4% to 26% of monochorionic, diamniotic twins. In TTTS, there is an unequal sharing of blood that passes between the twins through blood vessel connections on the surface of the placenta. One twin (the donor) pumps blood to the other twin (the recipient). The recipient twin receives an excessive amount of blood, leading to polyhydramnios, increased pressure, volume overload, and the release of mediators associated with hypertension. Conversely, the donor twin receives a low amount of blood, leading to oligohydramnios. The right ventricle of the recipient twin initially undergoes compensatory hypertrophy, followed by dilation and heart failure with tricuspid regurgitation and pulmonary stenosis and/or regurgitation, potentially progressing from functional to organic, with pulmonary atresia. Fetal hydrops due to heart failure can also occur. Some case reports describe the calcifications at both the pulmonary valve and trunk (supravalvular) levels in recipient twins [19–21]. The authors speculate that pulmonary artery thickening, and calcification may result from vascular injury caused by excessive volume load, as there is no pressure gradient between the aorta and pulmonary artery during fetal life.
GACI has been described in only two sets of monochorionic and dichorionic twins [22, 23]. In these cases, both twins were affected, with positive genetics for ENPP1 (ecto-nucleotide pyrophosphatase/phosphodiesterase).
Case report
Here we present our experience with a recipient twin from a monochorionic diamniotic twin pregnancy complicated by stage three TTTS, treated with fetoscopic selective laser ablation of placental anastomoses according to Solomon technique [24]. An echocardiogram 1 week after laser surgery showed moderate biventricular hypertrophy and dilatation, with a reduced caliper of the ascending aorta and pulmonary trunk. At birth, the echocardiogram revealed mild supravalvular pulmonary stenosis with mild stenosis of pulmonary branches, moderate supravalvular aortic stenosis, and dilatation of coronary arteries with small diffuse calcific nodules. Virological, metabolic, hormonal and autoantibody investigations were negative.
The degree of supravalvular aortic stenosis, characterized by a tubular appearance, hyperechogenicity, and thickening of the aortic wall, rapidly progressed during the first month. Additionally, significant calcification of the pulmonary trunk with severe pulmonary supravalvular stenosis, diffuse calcifications at the bifurcation and pulmonary branches, and diffuse nodular calcifications at the left coronary artery were also demonstrated (Figure 1). Computed tomography (CT) total body CT angiography [25] confirmed the echocardiographic supravalvular calcifications and excluded other vascular calcifications in the head, neck, thorax and abdomen vessels (Figure 2). The CT also revealed thickening of the wall of the abdominal aorta and renal arteries (Figure 2). The search for ENPP1 gene mutation (6q22) was negative. The mutation in ABCC6 (ATP Binding Cassette Subfamily C Member 6) gene was not tested because its link to GACI was unknown at the time of birth.
Figure 1
Echocardiogram. A – Severe pulmonary supravalvular stenosis, B – diffuse pulmonary supravalvular calcifications, C – coronary nodular calcifications, D – left and right ventricle hypertrophy
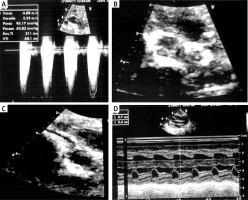
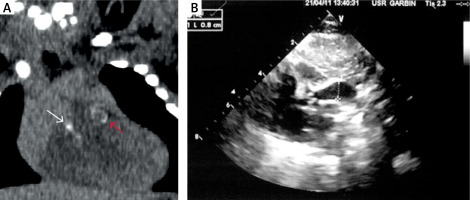
Figure 2
Computed tomography (CT) total body CT angiography. A – Coronal reformation from unenhanced CT. White and red arrows show supravalvular pulmonary and aortic calcifications. B – Aortic supravalvular tubular calcifications
Initially, β-blocker therapy was administered, but it resulted in only minimal reduction of supravalvular aortic and pulmonary gradients. Due to the severity of the stenoses, we, in agreement with the parents, initiated etidronate treatment (18 mg/kg/day). The drug was given after obtaining informed consent from parents, in galenic formulation provided by our hospital pharmacy. Monthly radiographic checks of the radius and ulna were performed to monitor for possible bone mass reduction. After 6 months of treatment, we observed mild residual supravalvular aortic stenosis and mild-to-moderate supravalvular pulmonary stenosis, both of which showed continuous improvement. An echocardiographic examination after 3 years showed minimal supravalvular aortic gradients with wall calcification and post-stenotic dilation. Additionally, there was mild supravalvular pulmonary stenosis with calcification of pulmonary branches, and the previously visible coronary artery calcific nodules were no longer present (Figure 3). Etidronate therapy was then discontinued with any side effects or reduction in bone mass. At 10-year follow-up, the echocardiographic findings remain unchanged.
Discussion
Stenosis of the pulmonary valve, up to atresia, has been demonstrated in several recipient twins in TTTS-complicated pregnancies. The recognized pathophysiology involves volume and pressure overload, predominantly affecting the right ventricle of the recipient twin. This overload occurs due to passage of hypertension mediators from the donor to the recipient, including renin, angiotensin and endothelin 1. The initial compensatory response of the right ventricle, characterized by progressive hypertrophy, itself contributes to reduced flow through the pulmonary valve, leading to functional stenosis or atresia. This functional form may progress to an organic one.
The vasoconstrictor and mitogenic effects of endothelin 1 may partly explain the increased thickness of the tunica intima of both the aorta and pulmonary artery, as observed in some recipient twins.
Literature reports on supravalvular pulmonary and aortic calcification, along with diffuse calcification of the pulmonary branches and aortic wall, consistently show negative genetics for both ENPP1 and ABCC6 mutations. These cases typically follow a benign course, with gradual resolution of the cardiovascular involvement in the absence of etidronate therapy.
However, in 1 case, aortic stenosis progression was observed despite therapy, leading to surgical intervention at the age of 4 months. Interestingly, both twins in that case showed the ABCC6 gene mutation [15]. This observation prompt us to hypothesize how epigenetic factors may influence the phenotype and the development (or lack) of calcifications.
Conversely, the renin-angiotensin-aldosterone system (RAAS) is involved in all stages of cardiovascular homeostasis, as demonstrated by Mahieu-Caputo et al. [26]. The renal expression of renin was up-regulated in donors and down-regulated in recipient twins, potentially serving as the link between abnormalities in fetal blood volume and vascular disturbances in TTTS. This initial positive adaptation then becomes deleterious, particularly in the recipient twin, who may be exposed to the transfer of various molecules produced by the donor via intertwin vascular anastomoses, including vasoactive RAAS hormones [27].
Some experimental studies have shown that chronic infusion of angiotensin II (AII) in rats increased the left ventricular mass, even when the pressor activity of AII was blocked [28]. In a rat model of “pressure-overload” cardiac hypertrophy (abdominal aortic constriction), treatment with an ACE inhibitor completely prevented the increase in the left ventricular mass, without effecting cardiac afterload [29]. These observations suggest that AII has a direct hypertrophic action on heart tissue, in addition to indirect effects mediated through increases in blood pressure and vascular resistance. All could be one of the most important epigenetic factors leading to the cardiovascular changes in the recipient twin, as observed in our patient. Conversely, studies performed on animal models of aortic valve stenosis (AS) show that the administration of angiotensin-receptor-blockers (ARBs) slows the progression of the disease compared to ACE inhibitors.
This difference might be explained by the presence of elevated levels of chymase in calcific aortic valve tissue. Similar to ACE, chymase converts AI to AII. Therefore, the inhibition of ACE activity may be bypassed by the action of chymase, resulting in the production of AII, while the effects of AII can be blocked by ARBs.
Several studies have been conducted to elucidate the processes involved in the progression of aortic valve stenosis, allowing us to hypothesize another epigenetic mechanism related to the described cases of diffuse aortic and pulmonary stenosis in TTTS twin recipients. Hong et al. [30] demonstrated how increasing concentrations of oxidized low-density lipoproteins (oxLDLs) lead to the uncoupling of endothelial nitric oxide synthase. This uncoupling results in a switch from nitric oxide (a protective antioxidant) production to the generation of reactive oxidative species (ROS) superoxide. Further lipid peroxidation and endothelial injury contribute to the inflammatory and calcification processes within the valve. The pro-inflammatory cytokines, coupled with reduced availability of nitric oxide, disrupt the balance between extracellular matrix production and breakdown in the valve [31, 32], ultimately leading to dystrophic microcalcification [33].
In conclusion, to the best of our knowledge, this is the first report of long-term follow-up of progressive arterial calcifications in a recipient twin from a TTTS-complicated pregnancy, without a positive genetic mutation. The patient was successfully treated with etidronate, thus avoiding the need for surgical intervention.
The mechanisms underlying the development of diffuse arterial calcifications in our patient extend beyond the known pressure-volume imbalance and in vasoactive RAAS hormones in the recipient twin. Significant epigenetic modifications affecting antioxidation processes may play a significant role.
Our experience, supported by clinical and laboratory data, demonstrates that etidronate effectively induces regression of arterial calcifications and is essentially free of major side effects.