Introduction
Smoking tobacco is a serious public health concern. It has been linked to increased risk of diseases, hospitalization, morbidity, and mortality [1, 2]. Namely, smoking a waterpipe (Wp) has been linked to the most devastating conditions including cardiovascular, immune, respiratory, and metabolic diseases [3]. The popularity of Wp smoking has regained momentum throughout the globe, especially among adolescents [4, 5]. In the Middle East, it has reached 9–16% in the Arab Gulf region [6], 13.4% in Sudan [7], 32.7% in the West Bank, 36.9% in Lebanon [8], 39% in Pakistan [9], and 66.3% in Jordan [4]. Similarly, it has reached 21.9% in Estonia, 22.7% in Latvia, 22.1% in the Czech Republic [8], 26% in the USA [10], and 46.7% in Poland [11]. This prevalence is rapidly increasing. In Jordan for example, Wp smoking has recently jumped ~8% (from 58.5% to 66.3%) in ~2 years, an alarming leap in such a short period in a rather vulnerable population [4, 12]. Interestingly, studies have shown that Wp smoking might lead to cigarette (Cg) smoking. A previous study [12] by Al-Sheyab et al. showed that Wp use during adolescence was a predictor of Cg smoking, confirming previous findings [13]. This is probably due to nicotine addiction [14], indicating that smoking one type of tobacco could lead to smoking another.
Waterpipe smoking involves inhaling smoke from charcoal-burned “Moasal” tobacco, after passing through a bowl of water [5]. The allure has been attributed mainly to social gathering, peer pressure, curiosity, novelty, aroma, flavor, publicity, acceptability, accessibility, and affordability, to name a few. However, misconceptions about the health effects of Wp smoking has been most prominent in alluring adolescents to smoke Wp [15–17].
Ferritin is a complex globular protein consisting of 24 subunits that acts primarily as a buffer system for iron deficiency and overload. It is found as a cytosolic protein in most body tissues to be a reservoir for iron. A small fraction of ferritin is released into the circulation to serve as an iron carrier; hence free iron is toxic to body cells and organs [18]. Ferritin reflects the total stored iron concentration, and thus has been used as an indirect marker for the total body stored iron and as a diagnostic test for iron-deficiency anemia [18]. Despite this physiological importance, elevation in ferritin has been linked to serious pathologies, particularly cardiometabolic [19–21] and respiratory [22] abnormalities.
Factors affecting ferritin level in humans are abundant, but lifestyle, such as exercise and diet, has been implicated. Factors that challenge oxygen delivery are believed to stimulate ferritin production and release [18]. Few studies, though, have reported that smoking Cg can elevate circulatory ferritin levels. An earlier study showed higher plasma ferritin levels in Cg smokers as compared to nonsmokers in the elderly [23]. Similarly, elevated serum ferritin level was associated with diminished lung function attributed to Cg smoking in a large Korean cohort (i.e. > 15,000 subjects) [22]. Ghio et al. also demonstrated increased circulatory iron, ferritin, along with transferrin saturation, in Cg smokers with and without COPD compared to never smokers [24]. The mechanism(s) for this increase is still elusive. However, smoking tobacco seems to cause an increase in body iron concentration, particularly in the lungs. Altered iron metabolism was attributed to oxidative stress and inflammation [24]. Given the toxic effect, freely floating iron stimulates ferritin production for safe iron storage [22].
Previous studies have shown differences in circulatory ferritin [20] and smoking type selection [4] between boys and girls. However, the effect of smoking Wp on circulatory ferritin is still unknown, in adolescents or even other populations. The gender-specific differences in circulatory ferritin in adolescents smoking tobacco (Cg or Wp) have also never been investigated. Therefore, the current study examined plasma ferritin concentration in boys and girls smoking Cg only, Wp only, or both (CgWp) versus never having smoked any type of tobacco. Additionally, the gender-specific differences in plasma ferritin in adolescents smoking tobacco were also determined. The results are important to understand the health effects of tobacco smoking, particularly on iron and ferritin metabolism. Furthermore, the study will shed light on the gender-specific differences in ferritin between boys versus girls smoking Cg only, Wp only, and CgWp.
Material and methods
Design and recruitment
The present data are part of the Irbid Tobacco Risk in Youth (Irbid-TRY) project, aimed at examining the health effects of Cg and Wp tobacco use among adolescents [4]. The current study is descriptive, cross-sectional, and comparative, designed to investigate the relationship of habitual tobacco smoking with plasma ferritin concentrations in adolescents.
Tobacco consumption status and plasma ferritin were obtained from apparently healthy adolescents in seventh-tenth grades (age range = 13–17 years) who were recruited from four boys’ schools and four girls’ schools (total = 8). All participating schools were public and governmental, located in Northern Jordan of the greater Irbid governorate. The students who reported, when asked, acute/chronic medical conditions, or being on medications/supplements that might affect plasma ferritin, were excluded from the study.
The study was approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology (JUST) and the Ministry of Education (MoE) in 2014/2015. JUST is located in Irbid, Northern Jordan, while the MoE is located in Amman, Jordan. After obtaining the ethics approval from the IRB committee at JUST, the legal guardians of the students approved the participation of their adolescent and signed the study consent form. In addition, students gave their assent as required by the ethics committees. All necessary measures were arranged, to assure safety and anonymity of the participants throughout the study phases. Study measurements were obtained under the supervision of qualified university professors.
Anthropometric measurements
Body weight (BW) was measured with a standard weighing scale, while height (HT) and waist circumference (WC) were determined with a standard measuring tape. The BW/HT2 formula was used to calculate the body mass index (BMI).
Tobacco smoking status
A validated Arabic version of the Youth Risk Behavior Survey (YRBS) was used to gather information on tobacco use among study participants. In this study, information on smoking Cg only, Wp only, or CgWp was obtained from the adolescents [25]. The survey asked: “Have you used Cg in the previous 30 days?”, and “Have you smoked WP in the past 30 days?”. The responses to these questions were described in previous publications from Irbid-TRY [4, 26]. Adolescents who indicated Cg, but not WP, smoking at any time in the past 30 days were considered as Cg only smokers, whereas adolescents who indicated Wp smoking but not Cg at any time in the past were considered Wp only smokers. A participant who smoked concurrently Cg and Wp tobacco was considered a dual (CgWp) smoker and was included in the analysis as such.
Blood sampling and plasma ferritin
For ferritin analysis, a total of 2 ml of blood was drawn from eligible participants in EDTA sterile tubes using standard venipuncture procedures. Blood samples were transferred in cold containers to medical laboratories at Jordan University of Science and Technology. Samples were centrifuged at 3000 × g for 5 min. Plasma samples were stored at 80°C until used. Levels of plasma ferritin were measured using the Access 2 Immunoassay System (Beckman Coulter, Atlanta, Georgia, USA).
Statistical analysis
Statistical analysis was computed using the SPSS statistical package (version 22.0; Chicago, IL). Data are expressed as means ± SD or percentages depending on type of investigated variable (continuous versus categorical). The α threshold was present at p < 0.05.
Whole sample and gender-stratified multiple linear regression were used to examine the relationship of age, gender, smoking status, family income, mother’s level of education, and father’s level of education, BMI, place of living, and family income with plasma ferritin. Subsequently, stepwise regression for the whole sample and gender-stratified regression were used to determine the relationships of type of tobacco use with plasma ferritin. Entire sample and gender-stratified ANCOVAs, after adjusting for confounders found related to plasma ferritin, were applied to analyze differences in plasma ferritin between the tobacco user groups (i.e. none versus Cg only, Wp only, and CgWp). LSD post-hoc analysis was used for subgroup comparisons.
Results
Participants
Self-reported smoking status was obtained from 2445 students while blood samples for plasma ferritin level were obtained from 1046 of these students. As presented in Table I, the total valid smoking status and plasma ferritin data were obtained for 849 boys (n = 470) and girls (n = 379) from the 7th–10th grade. The sociodemographic characteristics, including average age, weight, and height and proportion of genders, smoking type, family income level, and parent education level, are presented in Table I.
Table I
Demographic, smoking status, and plasma ferritin characteristics for the adolescents (n = 849)
| Parameter | % or mean (SD) |
|---|---|
| Demographics | |
| Gender – female (%) | 44.6 |
| Age [years], mean (SD) | 14.6 (1.1) |
| Weight [kg], mean (SD) | 56.6 (13.5) |
| Height [cm], mean (SD) | 161.1 (9.2) |
| BMI [kg/m2], mean (SD) | 21.7 (4.2) |
| Grade (%) | |
| 7th | 21.2 |
| 8th | 23.2 |
| 9th | 32.5 |
| 10th | 23.2 |
| Location (%) | |
| Rural | 53.8 |
| Urban | 47.2 |
| Family income (%)* | |
| Above poverty | 63 |
| Below poverty | 37 |
| Mother’s education level (%) | |
| Two-year/high school degree | 58.2 |
| Four-year/postgraduate degree | 41.8 |
| Father’s education level (%) | |
| Two-year/high school degree | 50.5 |
| Four-year/postgraduate degree | 49.5 |
| Smoking status (%) | |
| Never smoked cigarettes/waterpipe | 33.2 |
| Smoked cigarettes only | 8.8 |
| Smoked waterpipe only | 21.9 |
| Smoked waterpipe and cigarettes | 36.0 |
| Plasma ferritin [µg/l], mean (SD) | 15.5 (10.8) |
Relationship of smoking with plasma ferritin
The multiple linear regression for the whole sample showed that the model can predict (p < 0.0001; Df = 8) 8.2% of the variation in plasma ferritin. Further analysis showed that BMI (p < 0.001), gender (p < 0.001), smoking status (p < 0.02), place of living (p < 0.03), and family income (p < 0.02) were related to plasma ferritin. Subsequent stepwise regression for the entire sample revealed that BMI (p < 0.0001; Df = 1), gender (p < 0.0001; Df = 1), smoking status (p < 0.01; Df = 1), place of living (p < 0.004; Df = 1), and family income (p < 0.04; Df = 1) can predict 3.1%, 5.6%, 6.6%, 6.9% and 8.4%, respectively, of plasma ferritin level.
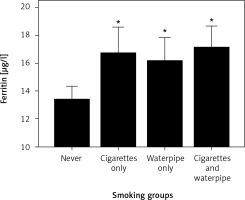
After adjusting for place of living, BMI, and family income, the 2-way (4 smoking groups * 2 genders) ANCOVA for the entire sample revealed a main effect for gender (p < 0.000; F = 16.5; Df = 1) and smoking status (p < 0.02; F = 3.2; Df = 3) but no interaction effect (p > 0.9; F = 1.0; Df = 3). As in Figure 1, post-hoc analysis showed greater plasma ferritin in the adolescents smoking Wp (p < 0.03; Df = 3) and CgWp (p < 0.004; Df = 3) versus the never smoked group. No differences (p > 0.05; Df = 3) were found in plasma ferritin among the adolescents smoking Cg versus Wp, CgWp, or never.
Gender differences in the relationship of smoking with plasma ferritin
The gender-stratified multiple linear regression showed that the model can predict (p < 0.0001; Df = 7) 7.1% of the variation in plasma ferritin among the boys. Further analysis showed that BMI (p < 0.003), smoking status (p < 0.05), place of living (p < 0.03), and family income (p < 0.05) were related to plasma ferritin in the boys. However, plasma ferritin was not related (p > 0.09; Df = 7) to any of the model components among the girls. Subsequent gender-stratified stepwise regression revealed that BMI (p < 0.004; Df = 1), smoking status (p < 0.03; Df = 1), and place of living (p < 0.004; Df = 1) can predict 2.8%, 3.9%, and 5.0%, respectively, of variation in plasma ferritin among the boys, while only BMI (p < 0.04; Df = 1) can predict 3.0% of variation in plasma ferritin in the girls.
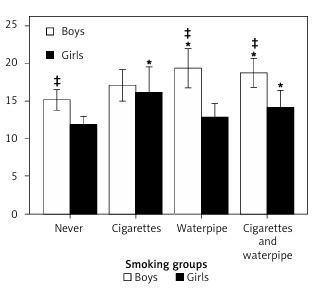
After adjusting for place of living and BMI, gender-stratified 1-way ANCOVA showed a main effect for smoking status in the boys (p < 0.02; F = 3.3; Df = 3) and girls (p < 0.03; F = 3.0; Df = 3). The post-hoc comparisons among the boys, shown in Figure 2, reveal greater plasma ferritin in the Wp (p < 0.006; Df = 3) and CgWp (p < 0.008; Df = 3) smoking groups versus the never smoking group, without a significant difference (p > 0.5; Df = 3) between Wp and CgWp smoking groups. No differences were found in plasma ferritin in the Cg smoking group versus never (p > 0.4; Df = 3), Wp (p > 0.2; Df = 3), or CgWp (p > 0.3; Df = 3) smoking groups.
Figure 2
Differences in plasma ferritin levels (μg/l) in the four smoking groups in the boys and girls. *P > 0.05 vs. never of same gender; ‡p > 0.05 vs. counterpart gender of the same smoking group
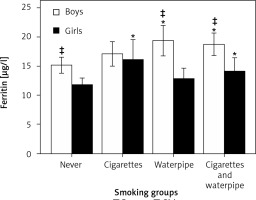
Figure 2 shows another subgroup comparison demonstrating greater plasma ferritin in the girls smoking Cg (p < 0.02; Df = 3) and CgWp (p < 0.024; Df = 3) versus the never smoking group, without a significant difference (p > 0.3; Df = 3) between Cg and CgWp smoking groups. No differences were found between the girls smoking Wp versus never (p > 0.3; Df = 3), Cg (p > 0.1; Df = 3), or CgWp (p > 0.3; Df = 3).
Discussion
The difference in ferritin metabolism between the boys and the girls smoking Cg versus Wp is unknown. The current study examined the relationship of smoking tobacco with plasma ferritin concentration. The results revealed greater plasma ferritin levels among the adolescents smoking Cg, Wp, and CgWp versus those who had never smoked. The current study confirms previous findings showing elevation in circulatory ferritin with smoking tobacco.
Increased ferritin with smoking has been shown in earlier studies. None of these studies, however, recruited adolescents or individuals smoking Wp; thus it is difficult to compare the current results. Previous studies have reported this increase in elderly smoking Cg [23] and Cg smokers with and without lung disease [22]. This elevation was associated with an upsurge of lung and systemic iron concentration in human and animal models [24].
The mechanism by which tobacco use increases blood ferritin levels might involve tissue inflammation and oxidative stress associated with smoking. For example, tobacco smoking, including Wp, has been shown to cause elevation in macrophages, lymphocytes, neutrophils [27], catalase, glutathione peroxidase, superoxide dismutase, TNF-α, IL-1β, IL-6, IL-10, and IL-12 [28]. This smoking-induced increase in oxidative and inflammatory stressors seems to cause tissue damage and subsequent leakage of ferritin from damaged cells [29], thus contributing to ferritin elevation [24]. Additionally, during smoking, CO and CO2 levels increase immediately, resulting in diminished affinity of red blood cells for oxygen, and subsequently compromised oxygen delivery to body tissue. As a compensatory response, these alterations in oxygen delivery result in increased intestinal iron absorption and circulatory levels [30], thereafter stimulating the production and release of ferritin.
The results also showed that plasma ferritin level is most elevated among the boys smoking Wp (i.e. Wp only and CgWp) as well as in the girls smoking Cg (i.e. Cg only and CgWp), another unprecedented finding that might suggest gender-specific alteration in ferritin by tobacco use types. Gender differences in the level of plasma ferritin between males and females in a normal population are well documented, with lower levels reported in females [20, 31–33]. These differences were attributed to female physiology and menstrual blood loss [33].
Given the sparsity of information about the effect of smoking, especially Wp, on ferritin, it is difficult to explain the gender differences in plasma ferritin according to smoking type. The observed differential modulation of ferritin level by smoking according to gender could be due to a combination of behavioral and physiological factors. For example, one study showed greater total puff volume and blood nicotine levels in men during Wp smoking [34]. In addition, gender differences in nicotine metabolism have been reported, which could suggest that girls might have the urge to smoke more often and more intensely to maintain sufficient circulatory nicotine via smoking Cg more often compared to Wp [35]. This is also supported by a study showing that women’s hormones accelerate cytochrome P450 2A6 activities, thus facilitating nicotine oxidation, which further explains faster nicotine metabolism and clearance [36].
Studies have also shown that charcoal burning during Wp smoking is associated with increased exposure to heavy metals, including iron, cadmium and arsenic [37], which might modify positively or negatively iron balance in the body. However, little is known about gender differences in nicotine and ferritin metabolism in adolescents smoking Cg versus Wp. Thus, more studies are needed to verify these mechanisms. Additionally, these findings need to be confirmed using longitudinal and intervention studies with a larger sample size to better characterize and interpret, and thus understand, gender-differential tobacco use.
It worth mentioning that differential modulations of ferritin among men and women also exist in other conditions. For example, in a study that examined the relationship between serum ferritin and vitamin D status, 25-hydroxyvitamin D level was inversely associated with ferritin levels in men, but was positively associated with ferritin levels in premenopausal women [38]. Another study showed that ferritin was positively associated with insulin resistance in men but not in premenopausal women [39]. Thus, the relationship between ferritin levels and gender seems to be complex and might be modulated differentially under various health conditions.
Ferritin is a protein essential for iron metabolism, including storage and delivery. Given its toxic effect, free-floating iron stimulates the production and release of ferritin to secure “safe” iron storage [18]. On the other hand, the elevation of ferritin is pathogenic and might predispose adolescents to a variety of disorders. It is associated with coronary artery disease [21], respiratory disease [22], the metabolic syndrome [19]; type 2 diabetes [40], obesity [20], and liver steatosis and fibrosis [41]. Additionally, it can predict acute myocardial infarction [42] and mortality in patients with multiple myeloma [43].
Implications: The results further confirm the negative effects of smoking on health. In this study, smoking seems to be a significant determinant of plasma ferritin levels in adolescents. Elevated ferritin level in smokers is linked to increased risk of many serious health problems, including cardiometabolic [19–21] and respiratory diseases [22]. Therefore, these results are particularly important and emphasize the need for greater efforts and more programs to tackle adolescent smoking. These efforts and programs should aim to minimize the negative effects and to limit the proliferation of smoking, particularly among adolescents. In addition, given that ferritin levels are commonly used as a diagnostic test of iron storage, special precautions are needed when using ferritin as an indicator of iron deficiency in smoking adolescents; different measures should be used.
There are some limitations to be clarified. First, the cross-sectional design used herein limits making cause-effect inferences about the associations between ferritin levels and smoking. Moreover, the sample size is relatively small, which restricts the generalizability of the results to other populations. Additionally, smoking status was obtained using a self-reported questionnaire, which is less reliable than biological biomarkers (i.e. saliva/blood nicotine/cotinine levels) [44], especially among adolescents. Using ferritin in the current study is another limitation. Given that circulatory ferritin is an acute phase reactant protein, it may increase in response to many factors (e.g. inflammation); thus another iron-related biomarker might be more informative. Therefore, future longitudinal/intervention studies using a larger sample size while controlling for possible confounders are recommended. Additionally, future studies examining the relationship of nicotine/cotinine with ferritin level among adolescents are needed. Also, using other biomarkers of iron status (such as serum iron or total iron-binding capacity (TIBC)) may strengthen our results and verify any existing relationship between smoking and iron deficiency anemia in this population.
In conclusion, there is a scarcity of information about the effect of gender and smoking type on ferritin metabolism, especially among adolescents. This study examined plasma ferritin concentration in boys and girls smoking Cg only, Wp only, or both (CgWp) versus never smoked. The results of this study support previous studies suggesting an elevation in ferritin level in individuals smoking tobacco (i.e. Cg). Yet, three important unique findings are drawn from this study: first, the ferritin differences in Wp smokers; second, the gender-specific differences in ferritin according to smoking type; and third, these differences were found in the adolescents. Ferritin was elevated in the boys smoking Wp and in girls smoking Cg. These results are unprecedented; however, future longitudinal and interventional studies are needed to verify the current findings. Additionally, programs to reduce the negative health effects and to limit the proliferation of smoking among adolescents, especially Wp, are warranted.