Gastric cancer remains a significant global health burden, necessitating the exploration of novel therapeutic strategies to combat its high morbidity and mortality rates [1]. Natural compounds have drawn considerable interest in cancer prevention and treatment research. Gedunin, a bioactive substance obtained from the Indian neem tree (Azadirachta indica), has gained prominence due to its diverse pharmacological properties, such as anti-inflammatory, anti-microbial, and anti-cancer effects [2].
Previous studies have demonstrated the potential of Gedunin as an anti-cancer agent in different cancer models [3, 4]. While the precise impact of gedunin on apoptosis in gastric cancer and its underlying molecular mechanisms are not yet fully elucidated, the high-mobility group box 1 (HMGB1)/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway is crucial for gastric cancer development and progression. [5]. This study aims to explore gedunin’s therapeutic potential as an apoptosis inducer and HMGB1/PI3K/AKT signaling pathway inhibitor in a rat model of gastric carcinogenesis induced by MNNG. By uncovering the molecular mechanisms behind its anti-cancer effects, this research may contribute to the development of innovative therapeutic strategies for gastric cancer treatment.
Methods
Animal model
Fifty-four Wistar rats, aged 7–8 weeks, weighing about 180–200 g, were obtained from the animal facility of the University. The rats were housed individually in standard cages with a 12-hour light-dark cycle and controlled temperature (22 ±2°C) and humidity (50 ±5%). Ethical approval for the study was obtained from the Ethical Animal Experimentation Committee of the Yantai Hospital of Traditional Chinses Medicine (AWE-2020-047), and all procedures were done in line with national and international guidelines for animal care and use.
Chemicals and reagents
Methylnitronitrosoguanidine (MNNG), a known gastric carcinogen, was obtained from Shanghai Slack Laboratory Animal Co., Ltd. Gedunin, a natural compound with potential anticancer properties, was acquired from Tocris Bioscience. Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), Vitamin C and E, and glutathione peroxidase (GPx) assay kits were obtained from Cayman Chemicals.
Experimental design
The rats were randomly divided into six groups (n = 9). Group 1 served as the MNNG model group and received intragastric administration of MNNG (200 mg/kg body weight) dissolved in water at 10-day intervals for a total of six doses. Groups 2–4 received MNNG and intragastric administration of gedunin at concentrations of 1, 10, and 100 µg/kg body weight, respectively, also at 10-day intervals for a total of six doses. Group 5 received gedunin at the highest dose (100 µg/kg body weight) three times a week without MNNG doses, serving as a positive control group to assess the effects of gedunin alone. Group 6 was the control group and did not receive any treatment.
Immunohistochemical examinations
Stomach tissues were cleaned, examined, and fixed in formalin. After processing and embedding, 4–5 µm thick sections were stained with H&E and AB-PAS for morphology and mucin-secreting cells assessment. Immunohistochemistry using caspase-3, caspase-9, Bax, Bcl2, and β-actin antibodies was performed to evaluate apoptotic and anti-apoptotic protein expression.
Results
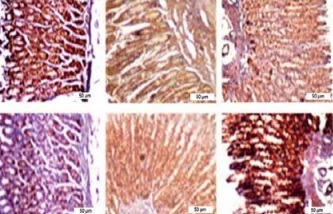
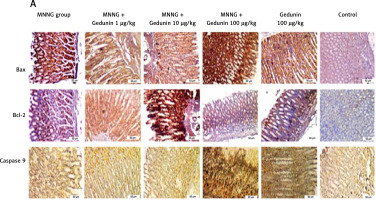
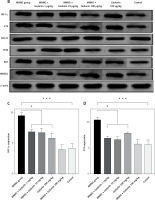
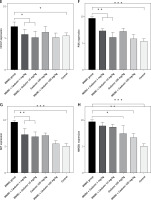
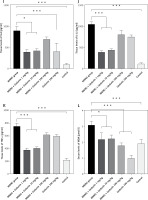
Figure 1 A demonstrates IHC staining results for caspase 9, Bax, and Bcl-2 proteins in gastric tissues. The study found that high-dose gedunin prevented MNNG-induced gastric adenocarcinogenesis and showed synergistic activity in inducing apoptosis. As shown in Figures 1 B–H, the protein levels of HIF-1α, AKT, PI3K, CT4, CD147, and HMGB1 proteins were significantly upregulated in MNNG-treated animals compared to the control group. As shown in Figure 1 I, the results showed that the serum levels of IL-1β, IL-6, and TNF-α significantly increased in MNNG-treated rats (group 1) compared to the control group. Additionally, treatment of rats with gedunin in groups 2 and 3 significantly reversed the increase in serum levels of inflammatory cytokines compared to the MNNG group. This finding indicates that gedunin has the ability to mitigate inflammation in response to MNNG-induced carcinogenesis. Results for cytokines obtained from analysis of serum and tissue samples showed that inflammation in MNNG rats (group 1) could be significantly reduced by high-dose gedunin supplementation (groups 4 and 5), and increasing the doses of gedunin lead to a decrease in the effects of these groups. The oxidative stress assay was carried out in serum and gastric tissues obtained from all experimental treatments; their TOS and TAS levels were measured, and the OSI value was calculated (Figure 1 J). The level of MDA was determined in gastric tissues homogenates by reaction with thiobarbituric acid and shown in Figure 1 K. The results confirmed that gedunin treatment effectively reduced oxidative stress levels. Do you need any further assistance with this information?
Figure 1
Gedunin induces apoptosis and inhibits HMBG1/PI3K/AKT signaling pathways in MNNG-induced gastric cancer, A – immunohistochemical examination of gastric tissues for the Bax, Bcl-2, and caspase 9. B–D – the protein levels of HIF-1α, CT4 in the gastric tissues of control and experimental groups. Data are expressed as mean ± SD (n = 9), *p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.001. E–H – the protein levels of CD147, PI3K, AKT, and HMGB1 in the gastric tissues of control and experimental groups. Data are expressed as mean ± SD (n = 9), *p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.001. I–K – the inflammatory cytokines (IL-6, IL-1β, and TNF-α) in the gastric tissues, L – MDA levels in the experimental rats. Unit used for MDA was nanomoles of MDA released per 100 mg of proteins. Data are expressed as mean ± SD (n = 9), *p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.001
Discussion
This study delved into the anticancer properties of gedunin in a rat model of MNNG-induced gastric carcinogenesis, unraveling the underlying molecular mechanisms. Gedunin emerges as a promising therapeutic candidate for Gastric cancer due to its ability to induce apoptosis, suppress the HMGB1/PI3K/AKT pathway, alleviate inflammation, and mitigate oxidative stress. Mechanistically, gedunin curtailed HMGB1 expression and hindered the activation of the downstream PI3K/AKT/HIF-1α signaling pathway. Notably, low-dose gedunin effectively prevented MNNG-induced gastric carcinogenesis.
Histopathological analysis revealed that gedunin treatment significantly reduced the incidence of gastric carcinoma compared to MNNG-treated rats. These findings align with previous studies demonstrating gedunin’s ability to suppress tumor growth and improve histopathological features in various cancer models [2, 3]. In this study, gedunin treatment was found to induce apoptosis in gastric cancer cells, as evidenced by the upregulation of caspase 9 and Bax proteins and downregulation of Bcl-2 protein.
The present study provides compelling evidence for the biphasic effect of gedunin on oxidative stress, inflammation, and apoptosis in MNNG-induced gastric carcinogenesis. Low doses of gedunin (1 and 10 mg/kg) significantly ameliorated MNNG-induced deleterious effects, demonstrating its potential as a chemopreventive agent against gastric cancer. Interestingly, the administration of a high dose of gedunin (100 mg/kg) yielded pro-oxidant and pro-inflammatory effects, exacerbating MNNG-induced damage. Possible explanations include saturation of detoxifying pathways at high concentrations, leading to accumulation of reactive species and subsequent oxidative stress [3].
In this study, gedunin treatment led to the downregulation of HMGB1, PI3K, and AKT protein expression levels. These findings suggest that gedunin may inhibit the HMGB1/PI3K/AKT signaling pathway, which has been associated with cell survival, proliferation, and metastasis in various cancers [6, 7]. The inhibition of this pathway by gedunin may contribute to its anti-cancer effects by suppressing tumor cell growth and promoting apoptosis. In comparison to previous studies, this study provides novel insights into the anti-cancer effects of gedunin in gastric carcinogenesis. It expands our understanding of gedunin’s mechanisms of action by demonstrating its ability to induce apoptosis, inhibit the HMGB1/PI3K/AKT signaling pathway, attenuate inflammation, and reduce oxidative stress [3, 6, 8].
The study demonstrated that gedunin treatment significantly reduced the levels of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, and TGF-β. These findings are consistent with previous studies showing that gedunin possesses anti-inflammatory properties by modulating the production of pro-inflammatory cytokines [9]. The attenuation of inflammation by gedunin may contribute to its anti-cancer effects by creating an unfavorable microenvironment for tumor growth.
In conclusion, this study investigates the relationship between gedunin dosage and gastric carcinogenesis in a rat model. The study found that gedunin exhibits a U-shaped dose-response relationship, where low doses have a protective effect against tumor growth and high doses may promote tumor growth.