Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
HEMATOLOGY / RESEARCH PAPER
Fresh autologous blood transfusion reverses erythrocytes damage by reducing oxidative stress via promoting mitochondrial metabolism and M2 macrophages polarization
1
School of Clinic Medicine, Ningxia Medical University, Ningxia 750004,China, China
2
Postgraduate Training Base in Shanghai Gongli Hospital, Ningxia Medical University, Shanghai 200135, China, China
3
Department of Anesthesiology, Shanghai Gongli Hospital, Naval Military Medical University, Shanghai 200135, China., China
4
Department of Anesthesiology, Lihuili Hospital, Medical School of Ningbo University, Zhejiang, 315040, China, China
Submission date: 2022-06-23
Final revision date: 2022-08-12
Acceptance date: 2022-09-13
Online publication date: 2022-10-03
Corresponding author
Jian-rong Guo
Department of Anesthesiology, Shanghai Gongli Hospital, Naval Military Medical University, Shanghai 200135, China., China
Department of Anesthesiology, Shanghai Gongli Hospital, Naval Military Medical University, Shanghai 200135, China., China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Systemic inflammatory and immune suppressive incidents constitute a significant hallmark of red blood cell transfusion's adverse effects. These adverse side effects are seemingly associated with old blood transfusion; therefore, we aim to investigate the underlying mechanism of fresh autologous blood transfusion (fABT) and its subsequent effect in diabetic mice.
Material and methods:
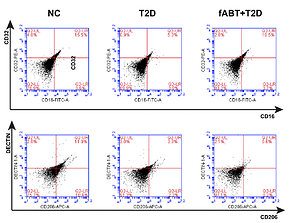
In the present study, we utilized 60 Swiss male mice aged 6-8 weeks, categorized into normal, diabetic, and freshly transfused with autologous blood. After treating the mice accordingly, further experimentations took place as we assessed the M1/M2 macrophage polarization concerning CD16/CD32/CD206 cells by flow cytometry, determined the mitochondrial metabolism using Lowry's analysis, measured hepatic oxidative stress using MDA and SOD assays, and examined the erythrocytic oxygen-carrying capacity (Q value) and oxygen consumption rate (OCR) and osmotic fragility.
Results:
Results showed that fresh autologous blood transfusion markedly reduced M2 macrophage polarization, enhanced hepatic mitochondrial metabolism, reduced hepatic oxidative stress, and promoted red blood cells' oxygen-carrying capacity while reducing its osmotic fragility and oxygen consumption. Moreover, we found that fABT promoted the IGF2/PI3K signaling pathway, These results indicate the vital necessity of providing fresh autologous blood transfusion during therapy or surgeries.
Conclusions:
In conclusion, our results are the first to highlight the underlying mechanism in which how fABT modulates physiological patterns in diabetic animals model, proposing a sound basis for utilizing fABT in clinical applications.
Systemic inflammatory and immune suppressive incidents constitute a significant hallmark of red blood cell transfusion's adverse effects. These adverse side effects are seemingly associated with old blood transfusion; therefore, we aim to investigate the underlying mechanism of fresh autologous blood transfusion (fABT) and its subsequent effect in diabetic mice.
Material and methods:
In the present study, we utilized 60 Swiss male mice aged 6-8 weeks, categorized into normal, diabetic, and freshly transfused with autologous blood. After treating the mice accordingly, further experimentations took place as we assessed the M1/M2 macrophage polarization concerning CD16/CD32/CD206 cells by flow cytometry, determined the mitochondrial metabolism using Lowry's analysis, measured hepatic oxidative stress using MDA and SOD assays, and examined the erythrocytic oxygen-carrying capacity (Q value) and oxygen consumption rate (OCR) and osmotic fragility.
Results:
Results showed that fresh autologous blood transfusion markedly reduced M2 macrophage polarization, enhanced hepatic mitochondrial metabolism, reduced hepatic oxidative stress, and promoted red blood cells' oxygen-carrying capacity while reducing its osmotic fragility and oxygen consumption. Moreover, we found that fABT promoted the IGF2/PI3K signaling pathway, These results indicate the vital necessity of providing fresh autologous blood transfusion during therapy or surgeries.
Conclusions:
In conclusion, our results are the first to highlight the underlying mechanism in which how fABT modulates physiological patterns in diabetic animals model, proposing a sound basis for utilizing fABT in clinical applications.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.