Introduction
Familial Mediterranean fever (FMF) is a common hereditary, auto-inflammatory disease, which is significantly associated with several ethnicities in the Mediterranean region, chiefly Turkish, Jewish, and Arabic communities [1].
Familial Mediterranean fever is an autosomal recessive disease that results from a point mutation in the Mediterranean fever gene (MEFV), located on the short arm of chromosome 16 [2]. Various mutations of the MEFV gene exist, and genotype-phenotype correlation studies have demonstrated a more severe disease course in individuals who are homozygous for the M694V mutation [3].
Schizophrenia is a chronic psychiatric disorder characterised by a heterogeneous presentation of negative and positive symptoms, which results in disruption of patients’ thoughts and emotions and interferes with the individuals’ capacity to engage in social events and to develop meaningful relationships [4]. Studies of neurological manifestations in paediatric FMF patients have found a significant association with various neurological disorders such as headache, epilepsy, pseudotumor cerebri, tremor, and multiple sclerosis, although several of these conditions are relatively rare [5].
Familial Mediterranean fever patients suffer from greater emotional stress in comparison to healthy patients, thus further adding to the morbidity of this patient population. Recent data indicate that FMF patients have considerably higher risk of developing depression and anxiety, which adversely affects patients’ quality of life [5–7]. Indeed, disease management using interleukin (IL)-1 antagonists led to significant improvement in physical function, role limitation due to emotional problems, emotional well-being, social functioning, and quality of life [8].
Clinical overlaps exist between these conditions. For instance, Eaton et al. [9] investigated the co-occurrence of autoimmune conditions with schizophrenia and demonstrated an increased occurrence of certain autoimmune conditions amongst schizophrenic patients, thus suggesting a shared genetic diathesis. Moreover, genome-wide association studies (GWAS) have identified several single nucleotide polymorphisms (SNPs) in schizophrenia that are primarily implicated in immune and inflammatory response [10].
However, the relationship between FMF and schizophrenia remains to be elucidated. In our current investigation, we aimed to assess the association between these disorders in a real-life population by utilising the Clalit Health Services (CHS) database, the largest Health Maintenance Organisation (HMO) in Israel.
Material and methods
This study was designed as a case-control study based on the chronic disease registry of the CHS, the largest integrated health service in Israel, which provides healthcare to more than 4,400,000 insured enrolees (roughly 50% of the Israeli population).
Data presented in the CHS database undergo a series of verification processes, which includes comparison of diagnoses from various sources. The validity of the data was verified in previously published studies [11, 12]. Wide-scale epidemiological studies can be conducted in real time on heterogeneous groups by using data-mining techniques. Familial Mediterranean fever patients were identified as patients with at least one diagnosis of FMF in their medical records, either provided by a general practitioner, a primary care physician, or a specialist. Familial Mediterranean fever has ethnic prediction to nations of the Mediterranean region. CHS entails a large database of FMF patients including approximately 7700 patients. The control group consisted of randomly selected CHS enrolees, with the exclusion of patients with an established diagnosis of FMF. Controls were age- and sex-matched to cases. Similarly, the diagnosis of schizophrenia included patients with these diagnoses in their medical records as entered by specialists in the CHS registry.
Additional data collected from the CHS database included age, gender, socioeconomic status (SES), body mass index (BMI), and smoking status. SES was defined according to the poverty index of the member’s residence area. The poverty index is computed from several parameters including household income, education, as well as other factors. The composite index ranged from 1 to 20, with 1 as the lowest SES.
The study was approved by the Ethical Committee of CHS, located at the Soroka Medical Centre, Beer-Sheva, Israel.
Statistical analysis
The occurrence of schizophrenia and was compared between FMF patients and controls in the study sample. The χ2 test was used to assess the distribution of categorical variables, while the t-test and one-way analysis of variance (ANOVA) were applied for continuous variables. Moreover, the association between FMF and schizophrenia was assessed using a multivariate logistic regression model controlling for possible confounding factors.
Analyses regarding survival were performed using Kaplan-Meier curves, the log-rank test, and the multivariate Cox proportional-hazards method to detect factors associated with increased all-cause risk mortality, with adjustment for risk factors where appropriate.
Statistical analysis was performed using the commercial software Statistical Package for Social Sciences for Windows (SPSS version 24.0, IBM, USA). Figures with a p-value less than 0.05 were considered statistically significant.
Results
Baseline characteristics of the study population and prevalence of schizophrenia
The present study included a total of 17,827 subjects, of whom 7747 were FMF patients and 10,080 were age- and sex-matched controls (case-control match 1 : 1.3). The average age of the population was 38.4 years, and 50.5% of the total population were females. No significant difference was demonstrated with regard to age, gender, and BMI between the case and control groups. Two differences of note between the case and control groups was the higher rates of smoking and of SES amongst the FMF group. Fifty FMF patients (0.6%) also had a diagnosis of schizophrenia, whereas amongst the healthy population, 89 patients (0.9%) had schizophrenia. The variance in schizophrenia between the case and control group was considered non-significant. The mortality rate among the whole population was 4.0%: 3.4% in the control group and 4.7% in the case group (p < 0.001). The basic characteristics of the study population are further detailed in Table I.
Table I
Overall population, familiar Mediterranean fever patients (cases), and age-and-sex matched controls – basic characteristics
| Characteristic | All population (N = 17,827) | Controls without FMF (n = 10,080) | FMF patients (n = 7747) | Statistical significance (p-value) |
|---|---|---|---|---|
| Age [mean ±SD] | 38.43 ±19.62 | 37.69 ±19.55 | 39.38 ±19.68 | NS |
| Age at diagnosis [mean ±SD] | 26.41 ±18.41 | 25.67 ±18.35 | 27.37 ±18.45 | NS |
| Gender, female, n (%) | 9000 (50.5) | 5121 (50.8) | 3879 (50.1) | NS |
| BMI [mean ±SD] | 24.81 ±63.91 | 24.42 ±50.61 | 25.30 ±77.41 | NS |
| SES, n (%)a | 0.0054 (0.0200 for trend) | |||
| Low | 8370 (50.6) | 4729 (50.3) | 3641 (51.1) | |
| Medium | 5609 (33.9) | 3153 (33.5) | 2455 (34.5) | |
| High | 2548 (15.4) | 1524 (16.2) | 1024 (14.4) | |
| Smoking, n (%) | 5000 (28.0) | 2588 (25.7) | 2412 (31.1) | < 0.001 |
| Schizophrenia, n (%) | 139 (0.8) | 89 (0.9) | 50 (0.6) | NS |
| All-cause mortality, n (%) | 707 (4.0) | 341 (3.4) | 366 (4.7) | < 0.001 |
Independent predictors of schizophrenia
On the multiple logistic regression model, FMF (OR = 0.64, 95% CI: 0.44–0.92, p = 0.0173), age (OR = 1.02, 95% CI: 1.01–1.03, p = 0.0010), and smoking (OR = 2.84, 95% CI: 1.69–3.63), p < 0.0001) were all independently associated with schizophrenia. Additional details are included in Table II.
Table II
Multivariate logistic regression assessing covariates associated with schizophrenia
On the multivariate Cox proportional-hazards regression model (Table III), FMF diagnosis (HR = 0.62, 0.43–0.90, p = 0.0126) and smoking status (HR = 2.20, 95% CI: 1.52–3.20, p < 0.0001) were predictors of schizophrenia.
Table III
Multivariate Cox proportional-hazards regression assessing covariates associated with schizophrenia
Survival analysis
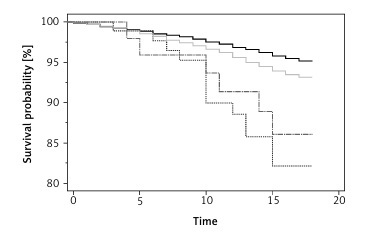
In the log-rank test, the Kaplan-Meier test results were statistically significant (χ2 = 48.30, degrees of freedom = 3, p < 0.0001; Figure 1 A), reflecting the impact of a schizophrenia diagnosis on FMF and non-FMF patients’ survival. In our analysis (Table IV), FMF patients had an increased all-cause mortality, as demonstrated when comparing FMF patients without schizophrenia to controls without schizophrenia (HR = 1.43, 95% CI: 1.23–1.66). Among the control group, schizophrenia was shown to significantly increase the HR as compared to patients without schizophrenia (HR = 3.97, 95% CI: 1.47–10.70). However, schizophrenia as a co-morbidity did not worsen the survival of FMF patients (HR = 2.17, 95% CI: 0.60–7.86]).
Figure 1
Kaplan-Meier survival plot of study cohorts (namely, familiar Mediterranean fever [FMF] cases with and without schizophrenia, and controls without FMF with and without schizophrenia)
Table IV
Hazard ratios (HRs) computed with their 95% confidence interval (CI) obtained from the Kaplan-Meier survival analysis
Independent predictors of mortality
In the Cox multivariate survival analysis (Table V), older age (HR = 1.08, 95% CI: 1.07–1.08, p < 0.0001), FMF diagnosis (HR = 1.36, 95% CI: 1.14–1.63, p = 0.0006), and schizophrenia (HR = 2.16, 95% CI: 1.24–3.76, p = 0.0063) were predictors of all-cause mortality. By contrast, female sex (HR = 0.73, 95% CI: 0.61–0.88, p = 0.0010) and high SES (HR = 0.0223, 95% CI: 0.56–0.96, p = 0.0223) were independently associated with better survival.
Table V
Cox multivariate survival analysis assessing covariates statistically associated with all-cause mortality
Discussion
To the best of our knowledge, this is the first study to demonstrate a lower rate of schizophrenia in FMF patients in comparison with controls. Moreover, although schizophrenia and FMF were both found to be independent predictors of all-cause mortality, schizophrenia did not appear to have a significant impact upon the survival of FMF patients.
The association of schizophrenia with other autoimmune diseases has been the focus of several studies in the past years. In a study performed on the Danish psychiatric register, Eaton et al. [9] found a 45% increase in the risk for schizophrenia for subjects with any autoimmune disease. Of the autoimmune diseases, five were more frequently associated with schizophrenia: thyrotoxicosis, celiac disease, acute haemolytic anaemia, interstitial cystitis, and Sjogren’s syndrome [9]. Nevertheless, certain autoimmune diseases did not increase the prevalence of schizophrenia. In a population-based cohort study on the Swedish population register, a significant reduction in the risk for rheumatoid arthritis (HR = 0.69, 95% CI: 0.59–0.80) and ankylosing spondylitis (HR = 0.62, 95% CI: 0.45–0.86]) were noted among schizophrenia patients [13]. Corroborating these findings, a recent meta-analysis, comprising 31 studies and pooling data from more than 25 million eligible individuals, demonstrated a positive association between certain non-neurological autoimmune disorders and psychosis but not with others. Thus Graves’ disease was positively associated with psychosis (OR = 1.33, 95% CI: 1.03–1.72), as was psoriasis (OR = 1.7, 95% CI: 1.29–2.84); however, rheumatoid arthritis (OR = 0.65, 95% CI: 0.50–0.84]) and ankylosing spondylitis (OR = 0.72, 95% CI: 0.54–0.98]) were not [14].
The mechanism driving the observed underlying association between certain autoimmune diseases and schizophrenia has been hypothesised to be multi-factorial in nature, involving inflammation and genetic predisposition, amongst other factors. Dysregulated inflammatory processes have been implicated in schizophrenia aetiology with various, albeit inconsistent findings with regard to cytokine levels as markers of schizophrenia [15–18]. In schizophrenia, higher levels of various cytokines have been reported, including IL-6, TNF-α, and IL-1β [19, 20]. Cytokine levels have been associated with structural brain changes, including decreased hippocampal volume and pre-cortex thinning, which is further associated with psychosis development [21, 22]. Finally, it is probable that schizophrenia shares a genetic diathesis with autoimmune diseases. HOPA (human opposite paired) gene on chromosome Xq13 encodes for T4 receptor, which when mutated has been linked to hypothyroidism and schizophrenia [23–25].
If schizophrenia has been previously associated with inflammation and autoimmune disorders, how could this explain the protective effect of an FMF diagnosis demonstrated in our study? As mentioned before, it is known that the genetic loss of pyrin in FMF results in enhanced IL-1β and IL-18 release, and thus excess inflammation. Another aspect of this inflammatory process can be attributed to the difference in the immune pathways activated in auto-inflammatory conditions, as compared with autoimmune pathophysiology. In the former, the innate immune system is activated, resulting in direct tissue inflammation; however, in the latter, the consequent activation of the adaptive immune system results in autoantibody and T-cell production [26]. It is plausible that this differential activation of the various arms of the immune system would partly explain the negative association between FMF and schizophrenia in our study results.
Exploring the genetic factors behind the occurrence of both FMF and schizophrenia could provide an avenue for understanding the decreased risk of disease occurrence. GWAS demonstrated a significant association of the major histocompatibility complex and SNPs in schizophrenia patients [27]. Recently, negative SNP genetic correlation was documented between schizophrenia and rheumatoid arthritis (p = 0.036), a result that is consistent with the epidemiological data reported on the negative association between the two disease entities, suggesting the prominence of genetic factors in both of the diseases’ aetiologies [28]. Moreover, the MHC I haplotype HLA-B*08, a structure reported to predispose to rheumatoid arthritis, has been shown to confer a decreased risk of schizophrenia [29]. Although FMF is a prototypical monogenic auto-inflammatory condition, polymorphisms in HLA loci have been reported to influence the genetic and phenotypic disease penetrance [30]. With regard to schizophrenia, it is probable that the differential effect of MHC polymorphism could confer protective effects in patients with FMF, a finding that needs to be confirmed with GWAS. Taken together, it seems that in conditions defined as pure autoimmune conditions (conditions associated with MHC class II) a positive association with schizophrenia is demonstrated [13]. In contrast, immunological conditions defined as mixed pattern disease (e.g. ankylosing spondylitis), polygenic auto inflammatory diseases (e.g. inflammatory arthritis presenting as rheumatoid arthritis), and monogenic auto-inflammatory diseases (e.g. FMF) offer a protective effect against schizophrenia. Our study is the first to demonstrate this relationship in auto-inflammatory conditions and corroborates the findings in the other immunological disease subgroups.
With regard to mortality, our analysis showed that schizophrenia increased all-cause mortality in comparison with controls. In a large meta-analysis on schizophrenia patients the median standardised mortality ratio for all persons for all-cause mortality was 2.58, suggesting an increased mortality risk in comparison to the general community [31].
Furthermore, FMF also had an increased mortality rate in comparison with healthy counterparts, an association that was the focus of investigation in a nationwide study, which demonstrated increased mortality in FMF male (HR = 1.71, 95% CI:1.06–2.75) and female (HR = 2.48, 95% CI: 1.03–5.99) patients. According to the findings of this study, the primary cause of death was renal amyloidosis, which accounted for 35% and 60% of deaths in men and women, respectively [32].
Our study has multiple strengths, including analysis of data in a real-life setting, which subsequently mirrors existing currents in the population. Moreover, the CHS database has one of the largest samples of FMF patients worldwide. However, several limitations warrant consideration, namely the observational design and lack of information regarding the temporal relationship between the conditions, which consequently precludes the determination of a causal relationship between FMF and schizophrenia. However, the reliability of CHS registry diagnosis has been alluded to by several studies and undergoes several levels of verification [11, 12]. Furthermore, information regarding disease duration and medications used to treat the conditions was not accessible.
In conclusion, our analysis revealed a reduced prevalence of schizophrenia in patients with FMF, suggesting a possible protective effect. Diagnosis with either FMF or schizophrenia was associated with an independent prediction of mortality. It seems that pure autoimmune conditions are positively associated with schizophrenia, whereas auto-inflammatory conditions confer a protective effect against schizophrenia. A better understanding of the genetic predispositions of both schizophrenia and FMF may help shed light on the pathophysiology and eventually assist in identifying better management and treatment options for both diseases.