In the current classification of the World Health Organization, gliomas are classified into four grades (G1–4). The most malignant in this group of central nervous syndrome (CNS) tumors is glioblastoma multiforme (GBM), constituting 12–15% of all brain tumors. Despite advances in neurosurgery, radiation and chemotherapy, the average survival rate is only 12.1 months in the application of radiotherapy or 14.6 months for additional therapy with temozolomide [1]. The previous decade has been a period of particular interest in cancer stem cells, identified in a variety of primary tumors – for example in cancers of the colon, lungs, and in some hemato-oncological diseases, e.g. chronic myeloid leukemia [2, 3].
Stem cells in GBM may be isolated from other cells in the primary tumor of the brain with the use of markers of neural stem cells, i.e. CD133, nestin, βIII-tubulin, as well as markers of glial cells, for example GFAP. Currently, it is being suggested that transcription factors such as Nanog, Oct-4, Rex and Olig2 can be used as biomarkers of cancer stem cells and help identify these cells in a tumor [4–6].
Although we know a number of stem cell markers, including GBM cell markers, much less is known about the behavior of those cells under the influence of radiation and chemotherapy, and their effect on tumor recurrence and overall survival. Moreover, the results of individual studies tend to be divergent. Some authors argue that the expression of markers of stem cells/progenitor cells in GBM is an unfavorable prognostic marker [7]. Other researchers disagree [8]. Therefore, the aim of the present work was to evaluate the relationship between expression of markers of stem/progenitor cells in the tumor and the time to tumor recurrence and the survival of patients with GBM.
The study involved material containing cancer tissue collected during routine neurosurgical treatment of 21 patients of both genders (10 women, 11 men) aged 30 to 77 years (median 56 years). The study protocol did not include another procedure during surgery or collection of more material than necessary for tumor removal. Nervous tissue comprising cancer tissue was frozen directly after removal in liquid nitrogen at –80°C. Once the diagnosis was confirmed by histopathological examination, detection of stem/progenitor cells was performed using molecular assessment. Patients were divided into groups according to the results of analysis in which the expression of markers of stem/progenitor cells was related to:
time to the recurrence of GBM: less than 6 months – 5 patients vs longer than 6 months – 5 patients.
overall survival: less than 12 months – 12 patients vs. equal to or longer than 12 months – 6 patients (3 patients were excluded from the analysis due to a lack of data regarding survival).
Total RNA obtained from the tumor tissue fragment was isolated using a TRIzol kit plus an RNA Purification Kit (Life Technologies, USA). 0.5 g of RNA was subjected to reverse transcription process using a First Strand cDNA Synthesis Kit (Thermo Scientific, USA). Analysis of expression of target genes and the housekeeping gene β-actin was performed using the apparatus CFX96 Real Time System (Bio-Rad, USA). In the reaction, we used primers specific for the tested genes, the cDNA template and a ‘mix’ containing SYBR Green – IQ SYBR Green Supermix (Bio-Rad, USA). Relative quantification of mRNA expression of genes tested was calculated using the comparative Ct method. As most of the analyzed continuous variables had statistical distributions significantly deviating from the normal distribution (Shapiro-Wilk test), analysis of data was performed using non-parametric tests. The significance of differences in quantitative variables between the two groups was performed using the Mann-Whitney U test, while the differences in qualitative variables were assessed with Fisher’s exact test. Quantitative values are presented as mean ± standard deviation. Statistical significance was assumed at p < 0.05. Calculations were performed using the Statistica StatSoft 10.0 software.
Identification of stem/progenitor cells in the GBM tissue was carried out on the basis of the expression levels of the genes CD133, GFAP, Nanog, Nestin, Oct-4, Olig2, Rex, βIII-tubulin using qRT-PCR. β-actin was used as the reference gene. In each case of GBM, we observed the expression of all the markers of stem cells.
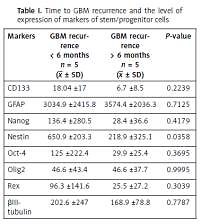
Therefore, the aim of this study was to determine whether there is a correlation between the expression of markers of neural stem cells and the time to tumor recurrence and survival of patients with GBM. First, the levels of expression of markers of neural stem cells were compared in patients with recurrent GBM. Those patients were divided into two subgroups, taking into account the time to tumor recurrence. A significantly shorter time to recurrence was observed in patients with higher expression of nestin (p = 0.0358) (Table I). According to some authors, nestin expression increases with malignancy of gliomas and correlates with survival of the patient [8, 9]. However, other investigators have not confirmed this correlation [5]. Due to the scarcity of the literature, elucidating the relation between nestin and the time to recurrence requires further detailed research.
Table I
Time to GBM recurrence and the level of expression of markers of stem/progenitor cells
A clear trend towards more rapid tumor recurrence was also observed in patients with higher expression of CD133, Nanog, Oct-4 and Rex. Similar correlations were observed between the survival of patients with GBM and the levels of expression of stem cell markers. As with the time to recurrence, overall survival was significantly shorter in persons with higher expression of nestin (p = 0.0440) (Table II). We also found a clear trend towards shorter survival for patients with higher expression of CD133, Nanog, Oct-4, Olig2 and Rex.
Table II
Survival time of patients with GBM and expression of markers of stem/progenitor cells
In conclusion it should be emphasized that despite a relatively small number of patients in this study we were able to identify cells in the GBM tissue which, as it may be presumed, are characterized by resistance to adjuvant therapy and therefore shorten the time to recurrence and the survival time of patients.
Using appropriate markers of neural stem cells, it can be assumed with high probability that these are GBM stem cells. This study showed that nestin proved to be a significant marker for those cells. Its role as a prognostic factor in the pathogenesis of GBM is also interesting, although the results of previous literature reports on this role of nestin are divergent.
Nestin is a class VI intermediate filament protein which may be re-expressed during the repair but also in the pathology of the central nervous system. Its clear expression has been observed in proliferating endothelial cells [10]. The close connection between nestin and neoangiogenesis in cancer may be conducive to more aggressive GBM, manifested in faster recurrence and shorter survival. According to our research, nestin expression was significantly higher in tumors that relapsed within a short time, as well as in patients with shorter survival.
Defining the specific markers of GBM and the determination of their clinical significance in the prognosis with regard to time to recurrence and survival, including response to treatment, may help in creating individual therapeutic strategies aimed at eliminating cancer stem cells in GBM.