Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
OBSTETRICS AND GYNAECOLOGY / BASIC RESEARCH
Expression of ADAMTS13 in human endometrium and its potential role in recurrent pregnancy loss
1
The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Submission date: 2020-06-12
Final revision date: 2021-02-07
Acceptance date: 2021-02-07
Online publication date: 2021-03-21
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The mechanisms underlying the pathogenesis of recurrent pregnancy loss (RPL) remain poorly known and effective approaches to treat this disease are lacking. Proteinases of the ADAMTS family play important roles in embryonic growth and development. Our previous study suggested a role of ADAMTS13 during pregnancy. The current study aimed to determine the expression of ADAMTS13 in human endometrium and its association with RPL.
Material and methods:
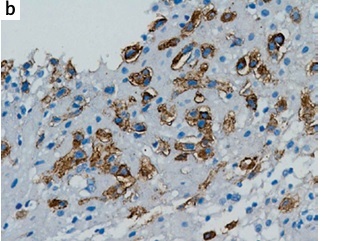
The spatiotemporal expression of ADAMTS13 in human endometrium was examined by immunohistochemistry. Real-time PCR and Western blot were then employed to determine the mRNA and protein expression levels of ADAMTS13 in human endometrium. Proteolytic cleavage of FRETS-VWF73 was performed to determine the activity of ADAMTS13 in plasma and that secreted by human endometrium. ELISA was carried out to measure plasma VWF antigen.
Results:
We found that proteolytically active ADAMTS13 is expressed in human endometrium throughout the menstrual cycle and pregnancy. The decidual expression levels of mRNA and protein in women with RPL were significantly lower compared with women with uncomplicated pregnancies (p < 0.01, p < 0.05, respectively). Furthermore, significantly reduced plasma ADAMTS13 activity (median (range) 69.09% (65.2–93.7%) vs. 93.62% (88.1–115.6%), p <0.001) and elevated plasma VWF antigen levels (median (range) of 125.5% (54.2–262.8%) vs. 91.9% (80.4–138.7%), p < 0.01) were detected in RPL patients compared with the control group.
Conclusions:
These findings suggest that ADAMTS13 may play a role in embryo implantation and the pathogenesis of recurrent pregnancy loss. Further investigation on ADAMTS13 gene knockout animal models is necessary for understanding the molecular mechanisms of the biological roles of ADAMTS13 during gestation.
The mechanisms underlying the pathogenesis of recurrent pregnancy loss (RPL) remain poorly known and effective approaches to treat this disease are lacking. Proteinases of the ADAMTS family play important roles in embryonic growth and development. Our previous study suggested a role of ADAMTS13 during pregnancy. The current study aimed to determine the expression of ADAMTS13 in human endometrium and its association with RPL.
Material and methods:
The spatiotemporal expression of ADAMTS13 in human endometrium was examined by immunohistochemistry. Real-time PCR and Western blot were then employed to determine the mRNA and protein expression levels of ADAMTS13 in human endometrium. Proteolytic cleavage of FRETS-VWF73 was performed to determine the activity of ADAMTS13 in plasma and that secreted by human endometrium. ELISA was carried out to measure plasma VWF antigen.
Results:
We found that proteolytically active ADAMTS13 is expressed in human endometrium throughout the menstrual cycle and pregnancy. The decidual expression levels of mRNA and protein in women with RPL were significantly lower compared with women with uncomplicated pregnancies (p < 0.01, p < 0.05, respectively). Furthermore, significantly reduced plasma ADAMTS13 activity (median (range) 69.09% (65.2–93.7%) vs. 93.62% (88.1–115.6%), p <0.001) and elevated plasma VWF antigen levels (median (range) of 125.5% (54.2–262.8%) vs. 91.9% (80.4–138.7%), p < 0.01) were detected in RPL patients compared with the control group.
Conclusions:
These findings suggest that ADAMTS13 may play a role in embryo implantation and the pathogenesis of recurrent pregnancy loss. Further investigation on ADAMTS13 gene knockout animal models is necessary for understanding the molecular mechanisms of the biological roles of ADAMTS13 during gestation.
REFERENCES (40)
1.
Toth B, Würfel W, Bohlmann M, et al. Recurrent miscarriage: diagnostic and therapeutic procedures. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry Number 015/050). Geburtshilfe Frauenheilkd 2018; 78: 364-81.
2.
Mohammad Seyedhassani S, Houshmand M, Mehdi Kalantar S, et al. BAX pro-apoptotic gene alterations in repeated pregnancy loss. Arch Med Sci 2011; 7: 117-22.
3.
Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol 2019; 14: 185-210.
4.
He X, Chen Q. Reduced expressions of connexin 43 and VEGF in the first-trimester tissues from women with recurrent pregnancy loss. Reprod Biol Endocrinol 2016; 14: 46.
5.
Benkhalifa M, Zayani Y, Bach V, et al. Does the dysregulation of matrix metalloproteinases contribute to recurrent implantation failure? Expert Rev Proteomics 2018; 15: 311-23.
6.
Fontanil T, Mohamedi Y, Cobo T, Cal S, Obaya AJ. Novel associations within the tumor microenvironment: fibulins meet ADAMTSs. Front Oncol 2019; 9: 796.
7.
Zhong S, Khalil RA. A Disintegrin and metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem Pharmacol 2019; 164: 188-204.
8.
Russell DL, Brown HM, Dunning KR. ADAMTS proteases in fertility. Matrix Biol 2015; 46: 54-63.
9.
Jokimaa V, Oksjoki S, Kujari H, Vuorio E, Anttila L. Altered expression of genes involved in the production and degradation of endometrial extracellular matrix in patients with unexplained infertility and recurrent miscarriages. Mol Hum Reprod 2002; 8: 1111-6.
10.
Uemura M, Tatsumi K, Matsumoto M, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood 2005; 106: 922-4.
11.
Mazepa MA, Park YA, Raval JS. Taking empiricism out of immune thrombotic thrombocytopenic purpura: current and future treatment strategies. Transfus Med Rev 2019; 33: 248-55.
12.
Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program 2018; 30: 530-8.
13.
Feng Y, Li X, Xiao J, et al. ADAMTS13: more than a regulator of thrombosis. Int J Hematol 2016; 104: 534-9.
14.
Xiao J, Feng Y, Li X, et al. Expression of ADAMTS13 in normal and abnormal placentae and its potential role in angiogenesis and placenta development. Arterioscler Thromb Vasc Biol 2017; 37: 1748-56.
15.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol 1975; 122: 262-3.
16.
Wu WX, Glasier A, Norman J, Kelly RW, Baird DT, McNeilly AS. The effects of the antiprogestin mifepristone, in vivo, and progesterone in vitro on prolactin production by the human decidua in early pregnancy. Hum Reprod 1990; 5: 627-31.
17.
Maslar IA, Ansbacher R. Effects of progesterone on decidual prolactin production by organ cultures of human endometrium. Endocrinology 1986; 118: 2102-8.
18.
Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost 2006; 4: 1396-404.
19.
Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014; 25: 605-20.
20.
Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005; 129: 93-100.
21.
Zhu H, Leung PC, MacCalman CD. Expression of ADAMTS-5/implantin in human decidual stromal cells: regulatory effects of cytokines. Hum Reprod 2007; 22: 63-74.
22.
Ng YH, Zhu H, Pallen CJ, Leung PCK, MacCalman CD. Differential effects of interleukin-1beta and transforming growth factor-beta1 on the expression of the inflammation-associated protein, ADAMTS-1, in human decidual stromal cells in vitro. Hum Reprod 2006; 21: 1990-9.
23.
Wen J, Zhu H, Murakami S, Leung PCK, MacCalman CD. Regulation of a disintegrin and metalloproteinase with thrombospondin repeats-1 expression in human endometrial stromal cells by gonadal steroids involves progestins, androgens, and estrogens. J Clin Endocrinol Metab 2006; 91: 4825-35.
24.
Wen J, Zhu H, Leung PC. Gonadal steroids regulate the expression of aggrecanases in human endometrial stromal cells in vitro. J Cell Mol Med 2013; 17: 1325-34.
25.
Shindo T, Kurihara H, Kuno K, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest 2000; 105: 1345-52.
26.
Mittaz L, Russell DL, Wilson T, et al. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod 2004; 70: 1096-105.
27.
Banno F, Kokame K, Okuda T, et al. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood 2006; 107: 3161-6.
28.
Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest 2005; 115: 2752-61.
29.
Madan P, Bridges PJ, Komar CM, et al. Expression of messenger RNA for ADAMTS subtypes changes in the periovulatory follicle after the gonadotropin surge and during luteal development and regression in cattle. Biol Reprod 2003; 69: 1506-14.
30.
Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res 2007; 120: 9.
31.
Peng Y, Chen Q, Yang T, Tao Y, Lu X, Liu C. Cultured mycelium Cordyceps sinensis protects liver sinusoidal endothelial cells in acute liver injured mice. Mol Biol Rep 2014; 41: 1815-27.
32.
Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta 2011; 12: 2.
33.
Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology 2000; 31: 75-82.
34.
Manea M, Tati R, Karlsson J, Békássy ZD, Karpman D. Biologically active ADAMTS13 is expressed in renal tubular epithelial cells. Pediatr Nephrol 2010; 25: 87-96.
35.
Manea M, Kristoffersson A, Schneppenheim R, et al. Podocytes express ADAMTS13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br J Haematol 2007; 138: 651-62.
36.
Lee M, Rodansky ES, Smith JK, Rodgers GM. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res 2012; 84: 109-15.
37.
Lee M, Keener J, Xiao J, Zheng XL, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci 2015; 72: 349-56.
39.
Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-regulated endometrial factors controlling implantation. Am J Reprod Immunol 2016; 75: 237-45.
40.
Billhaq DH, Lee SH, Lee S. The potential function of endometrial-secreted factors for endometrium remodeling during the estrous cycle. Anim Sci J 2020; 91: e13333.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.