Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / BASIC RESEARCH
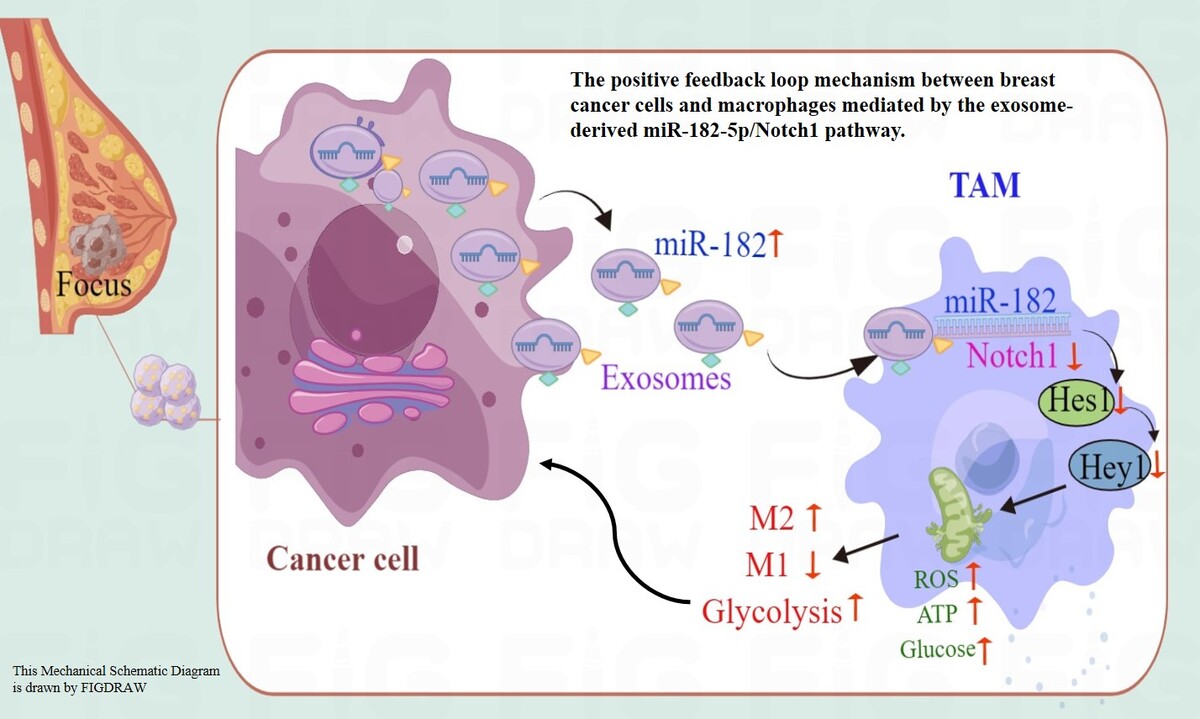
Exosomal miR-182-5p from breast cancer cells reprogram tumor-associated macrophages and promote triple-negative breast cancer progression by targeting Notch1 in macrophages
1
Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
2
The Clinical Medical Research Center of Breast and Thyroid Tumor in Xinjiang, Urumqi, Xinjiang, China
3
Xinjiang Key Laboratory of Oncology, Urumqi, Xinjiang, China
Submission date: 2024-02-08
Final revision date: 2024-03-30
Acceptance date: 2024-04-12
Online publication date: 2024-04-14
Corresponding author
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The regulatory role of miR-182 in breast cancer malignancy and macrophage reprogramming is well established. However, the mechanisms through which miR-182 overexpression in tumor cells influences macrophage polarization remain elusive.
Material and methods:
After transfection with miR-182-5p mimics, inhibitors, and controls for 24 h, exosomes were extracted by differential centrifugation from transfected MDA-MB-231. Macrophages were co-cultured with these exosomes to illustrate the regulative effects of exo-miR-182-5p reprogram macrophage. Furthermore, breast cancer cells were co-cultured with exo-miR-182-5p reprogrammed M2 macrophages to demonstrate the effects of reprogrammed M2 macrophages to influence breast cancer progression. After all, these findings were validated in a cell-derived xenograft (CDX) BALB/C nude-mouse model.
Results:
This study demonstrated that exosome-derived miR-182-5p from triple-negative breast cancer (TNBC) cells reprograms M2 macrophage polarization through direct combination with Notch1, thereby enhancing breast cancer progression in vitro and in vivo. When co-cultured with exosomes from TNBC cells transfected with miR-182-5p mimics or inhibitors, macrophages showed altered Notch1/Hes1 pathway expression, leading to M2 polarization and subsequent changes in reactive oxygen species (ROS), inflammation, and other biochemical markers. Furthermore, breast cancer cells co-cultured with exosome-reprogrammed macrophages exhibited increased colony formation, migration, and invasion, as well as reduced apoptosis. These findings were validated in a BALB/C nude-mouse model.
Conclusions:
This study pioneers the elucidation of the feedback loop mechanism between breast cancer cells and macrophages mediated by the exosome-derived miR-182-5p/Notch1 pathway, highlighting its role in macrophage reprogramming. Although the therapeutic application of miR-182-5p inhibitors as anticancer agents remains in the early stages, targeting macrophage polarization represents a promising avenue for breast cancer therapy.
The regulatory role of miR-182 in breast cancer malignancy and macrophage reprogramming is well established. However, the mechanisms through which miR-182 overexpression in tumor cells influences macrophage polarization remain elusive.
Material and methods:
After transfection with miR-182-5p mimics, inhibitors, and controls for 24 h, exosomes were extracted by differential centrifugation from transfected MDA-MB-231. Macrophages were co-cultured with these exosomes to illustrate the regulative effects of exo-miR-182-5p reprogram macrophage. Furthermore, breast cancer cells were co-cultured with exo-miR-182-5p reprogrammed M2 macrophages to demonstrate the effects of reprogrammed M2 macrophages to influence breast cancer progression. After all, these findings were validated in a cell-derived xenograft (CDX) BALB/C nude-mouse model.
Results:
This study demonstrated that exosome-derived miR-182-5p from triple-negative breast cancer (TNBC) cells reprograms M2 macrophage polarization through direct combination with Notch1, thereby enhancing breast cancer progression in vitro and in vivo. When co-cultured with exosomes from TNBC cells transfected with miR-182-5p mimics or inhibitors, macrophages showed altered Notch1/Hes1 pathway expression, leading to M2 polarization and subsequent changes in reactive oxygen species (ROS), inflammation, and other biochemical markers. Furthermore, breast cancer cells co-cultured with exosome-reprogrammed macrophages exhibited increased colony formation, migration, and invasion, as well as reduced apoptosis. These findings were validated in a BALB/C nude-mouse model.
Conclusions:
This study pioneers the elucidation of the feedback loop mechanism between breast cancer cells and macrophages mediated by the exosome-derived miR-182-5p/Notch1 pathway, highlighting its role in macrophage reprogramming. Although the therapeutic application of miR-182-5p inhibitors as anticancer agents remains in the early stages, targeting macrophage polarization represents a promising avenue for breast cancer therapy.
REFERENCES (51)
1.
Ostman A. The tumor microenvironment controls drug sensitivity. Nat Med 2012; 18: 1332-4.
2.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423-37.
3.
Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol 2022; 43: 523-45.
4.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022; 21: 799-820.
6.
Zhang B, Vogelzang A, Miyajima M, et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumor immunity. Nature 2021; 599: 471-6.
7.
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196: 254-65.
9.
Gharaibeh L, Elmadany N, Alwosaibai K, Alshaer W. Notch1 in cancer therapy: possible clinical implications and challenges. Mol Pharmacol 2020; 98: 559-76.
10.
Fung E, Tang SMT, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation 2007; 115: 2948-56.
11.
Kabłak-Ziembicka A, Badacz R, Okarski M, Wawak M, Przewłocki T, Podolec J. Cardiac microRNAs: diagnostic and therapeutic potential. Arch Med Sci 2023; 19: 1360-81.
12.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281-97.
13.
Weidle UH, Dickopf S, Hintermair C, Kollmorgen G, Birzele F, Brinkmann U. The role of micro RNAs in breast cancer metastasis: preclinical validation and potential therapeutic targets. Cancer Genom Proteomics 2018; 15: 17-39.
14.
Liu ZK, Xu H, Li X, Zhang RS, Bai JH, Zhang XF. Micro-RNA-325 targets lipocalin 15 to suppress proliferation, migration and invasion of breast cancer cells. Arch Med Sci 2020; 19: 1099-107.
15.
Xu YQ, Wang HL, Gao WK. MiRNA-610 acts as a tumour suppressor to depress the cisplatin resistance in hepatocellular carcinoma through targeted silencing of hepatoma-derived growth factor. Arch Med Sci 2019; 16: 1394-401.
16.
Golbakhsh MR, Boddouhi B, Hatami N, et al. Down-regulation of microRNA-182 and microRNA-183 predicts progression of osteosarcoma. Arch Med Sci 2017; 13: 1352-6.
17.
Chiang CH, Hou MF, Hung WC. Up-regulation of miR-182 by beta-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta 2013; 1830: 3067-76.
18.
Lei R, Tang J, Zhuang X, et al. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene 2014; 33: 1287-96.
19.
Yue D, Qin X. miR-182 regulates trastuzumab resistance by targeting MET in breast cancer cells. Cancer Gene Ther 2019; 26: 1-10.
20.
Sang Y, Chen B, Song X, et al. circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther 2019; 27: 1638-52.
21.
Ma C, He D, Tian P, et al. miR-182 targeting reprograms tumor-associated macrophages and limits breast cancer progression. PNAS 2022; 119: e2114006119.
22.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367: eaau6977.
23.
Han QF, Li WJ, Hu KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer 2022; 21: 207.
24.
Borkowska EM, Kutwin P, Rolecka D, Konecki T, Borowiec M, Jabłonowski Z. Clinical value of microRNA-19a-3p and microRNA-99a-5p in bladder cancer. Arch Med Sci 2020; 19: 694-702.
25.
Wang X, Huang J, Chen W, Li G, Li Z, Lei J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp Mol Med 2022; 54: 1390-400.
26.
da Costa VR, Araldi RP, Vigerelli H, et al. Exosomes in the tumor microenvironment: from biology to clinical applications. Cells 2021; 10: 2617.
27.
Xu Z, Chen Y, Ma L, et al. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol Ther 2022; 30: 3133-54.
28.
Liang G, Zhu Y, Ali DJ, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol 2020; 18: 10.
29.
Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/ progenitor cells and the estrogen receptor. Trends Endocrinol Metab 2004; 15: 193-7.
30.
Wang J, Fu L, Gu F, Ma Y. Notch1 is involved in migration and invasion of human breast cancer cells. Oncol Rep 2011; 26: 1295-303.
31.
Fu YP, Edvardsen H, Kaushiva A, et al. NOTCH2 in breast cancer: association of SNP rs11249433 with gene expression in ER-positive breast tumors without TP53 mutations. Mol Cancer 2010; 9: 113.
32.
Yamaguchi N, Oyama T, Ito E, et al. NOTCH3 signaling pathway plays crucial roles in the proliferation of ErbB2-negative human breast cancer cells. Cancer Res 2008; 68: 1881-8.
33.
Chen J, Imanaka N, Griffin JD. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer 2010; 102: 351-60.
34.
Abravanel DL, Belka GK, Pan TC, et al. Notch promotes recurrence of dormant tumor cells following HER2/neutargeted therapy. J Clin Invest 2015; 125: 2484-96.
35.
Mao J, Song B, Shi Y, et al. ShRNA targeting Notch1 sensitizes breast cancer stem cell to paclitaxel. Int J Biochem Cell Biol 2013; 45: 1064-73.
36.
Reedijk M, Odorcic S, Chang L, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 2005; 65: 8530-7.
37.
Baker A, Wyatt D, Bocchetta M, et al. Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast cancer cell proliferation and stem cell survival. Oncogene 2018; 37: 4489-504.
38.
Yuan X, Zhang M, Wu H, et al. Expression of Notch1 correlates with breast cancer progression and prognosis. PLoS One 2015; 10: e0131689.
39.
Xiao YS, Zeng D, Liang YK, et al. Major vault protein is a direct target of Notch1 signaling and contributes to chemoresistance in triple-negative breast cancer cells. Cancer Lett 2019; 440-441: 156-67.
40.
Liu Y, Liu N, Xu D, et al. Hsa-miR-599 inhibits breast cancer progression via BRD4/Jagged1/Notch1 axis. J Cell Physiol 2022; 237: 523-31.
41.
Rui X, Zhao H, Xiao X, Wang L, Mo L, Yao Y. MicroRNA-34a suppresses breast cancer cell proliferation and invasion by targeting Notch1. Exp Ther Med 2018; 16: 4387-92.
42.
Li HC, Chen YF, Feng W, et al. Loss of the Opa interacting protein 5 inhibits breast cancer proliferation through miR-139-5p/NOTCH1 pathway. Gene 2017; 603: 1-8.
43.
Wang B, Wang Y, Wang X, et al. Extracellular vesicles carrying miR-887-3p promote breast cancer cell drug resistance by targeting BTBD7 and activating the Notch1/Hes1 signaling pathway. Dis Markers 2022; 2022: 5762686.
44.
Chen X, Su C, Wei Q, Sun H, Xie J, Nong G. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate diffuse alveolar hemorrhage associated with systemic lupus erythematosus in mice by promoting M2 macrophage polarization via the micro-RNA-146a-5p/NOTCH1 axis. Immunol Invest 2022; 51: 1975-93.
45.
Moradi-Chaleshtori M, Shojaei S, Mohammadi-Yeganeh S, Hashemi SM. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci 2021; 282: 119800.
46.
Tong F, Mao X, Zhang S, et al. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett 2020; 478: 34-44.
47.
Chen J, Li Z, Yue C, et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying miR-1827 downregulate SUCNR1 to inhibit macrophage M2 polarization and prevent colorectal liver metastasis. Apoptosis 2023; 28: 549-65.
48.
Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol 2018; 16: 103.
49.
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release 2020; 318: 1-15.
50.
Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014; 6: a021857.
51.
Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma 2022; 9: 157-70.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.