Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
EXPERIMENTAL RESEARCH
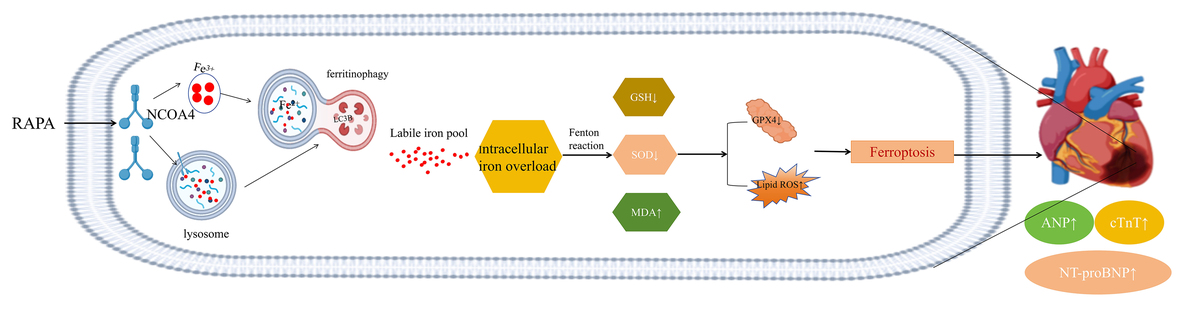
Excessive autophagy of myocardial cells promotes ferroptosis and exacerbates heart failure in the state
of myocardial infarction
1
Center for Xin'an Medicine and Modernization of Traditional Chinese Medicine of IHM, Anhui University of Chinese Medicine, Hefei, China
2
College of Traditional Chinese Medicine, Anhui University of Chinese Medicine, Hefei, China
3
School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, China
4
Key Laboratory of Xin’an Medical Education Department, Hefei, China
Submission date: 2024-02-22
Final revision date: 2024-05-02
Acceptance date: 2024-05-15
Online publication date: 2024-06-12
Corresponding author
KEYWORDS
ferritinophagyferroptosisautophagyglutathione peroxidase 4heart failurenuclear receptor coactivator 4
TOPICS
ABSTRACT
Introduction:
This study investigates the molecular mechanisms by which excessive autophagy exacerbates post-myocardial infarction heart failure (post-MI HF) through nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy and ferroptosis.
Material and methods:
We developed a post-MI heart failure model in Sprague-Dawley rats via coronary artery ligation, alongside an in vitro heart failure model using hypoxia/reoxygenation-stimulated H9C2 cells. Intervention with rapamycin (autophagy activator), 3-methyladenine (autophagy inhibitor), desferrioxamine, and ferredoxin-1 (ferroptosis inhibitors) was performed. Various techniques, including echocardiography, immunofluorescence colocalization, C11 BODIPY 581/591 staining, flow cytometry, transmission electron microscopy, western blotting, and RT-qPCR, were employed.
Results:
In vivo analyses revealed that NCOA4-mediated ferritinophagy and ferroptosis are significant in post-MI HF. Manipulating autophagy through rapamycin and 3-methyladenine influenced the expression of NCOA4 and glutathione peroxidase 4 (GPX4), subsequently affecting ferroptosis and modulating heart failure severity. Our in vitro experiments corroborated these findings, demonstrating that heightened autophagy amplifies NCOA4 expression, which in turn fosters ferroptosis and exacerbates myocardial injury. Interestingly, silencing of NCOA4 partially mitigated autophagy-induced iron deficiency, indicating a crucial intersection between autophagy and iron metabolism. Moreover, the cardioprotective effects observed following NCOA4 silencing were negated by concurrent GPX4 silencing.
Conclusions:
Our findings show that autophagy precedes NCOA4 in its regulatory pathway and directly influences ferritinophagy. Enhanced autophagy augments intracellular free iron and unstable iron pools, triggering lipid peroxidation through ferritinophagy, which promotes ferroptosis and impairs cardiac function. These insights offer a novel scientific basis for developing therapeutic strategies for post-MI HF.
This study investigates the molecular mechanisms by which excessive autophagy exacerbates post-myocardial infarction heart failure (post-MI HF) through nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy and ferroptosis.
Material and methods:
We developed a post-MI heart failure model in Sprague-Dawley rats via coronary artery ligation, alongside an in vitro heart failure model using hypoxia/reoxygenation-stimulated H9C2 cells. Intervention with rapamycin (autophagy activator), 3-methyladenine (autophagy inhibitor), desferrioxamine, and ferredoxin-1 (ferroptosis inhibitors) was performed. Various techniques, including echocardiography, immunofluorescence colocalization, C11 BODIPY 581/591 staining, flow cytometry, transmission electron microscopy, western blotting, and RT-qPCR, were employed.
Results:
In vivo analyses revealed that NCOA4-mediated ferritinophagy and ferroptosis are significant in post-MI HF. Manipulating autophagy through rapamycin and 3-methyladenine influenced the expression of NCOA4 and glutathione peroxidase 4 (GPX4), subsequently affecting ferroptosis and modulating heart failure severity. Our in vitro experiments corroborated these findings, demonstrating that heightened autophagy amplifies NCOA4 expression, which in turn fosters ferroptosis and exacerbates myocardial injury. Interestingly, silencing of NCOA4 partially mitigated autophagy-induced iron deficiency, indicating a crucial intersection between autophagy and iron metabolism. Moreover, the cardioprotective effects observed following NCOA4 silencing were negated by concurrent GPX4 silencing.
Conclusions:
Our findings show that autophagy precedes NCOA4 in its regulatory pathway and directly influences ferritinophagy. Enhanced autophagy augments intracellular free iron and unstable iron pools, triggering lipid peroxidation through ferritinophagy, which promotes ferroptosis and impairs cardiac function. These insights offer a novel scientific basis for developing therapeutic strategies for post-MI HF.
REFERENCES (44)
1.
Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators Inflamm 2017; 2017: 7018393.
2.
He X, Du T, Long T, Liao X, Dong Y, Huang ZP. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct Target Ther 2022; 7: 134.
3.
Sabouret P, Lemesle G, Bellemain-Appaix A, et al. Post-discharge and long-term follow-up after an acute coronary syndrome: International Collaborative Group of CNCF position paper. Arch Med Sci 2022; 18: 839-854.
4.
Wang H, Chai K, Du M, et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail 2021; 14: e008406.
5.
Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J 2022; 43: 2549-2561.
6.
Maejima Y, Zablocki D, Nah J, Sadoshima J. The role of the Hippo pathway in autophagy in the heart. Cardiovasc Res 2023; 118: 3320-3330.
7.
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014; 509: 105-109.
8.
Yu F, Zhang Q, Liu H, et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov 2022; 8: 40.
9.
Fang Y, Chen X, Tan Q, Zhou H, Xu J, Gu Q. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Cent Sci 2021; 7: 980-989.
10.
Santana-Codina N, Gikandi A, Mancias JD. The role of NCOA4-mediated ferritinophagy in ferroptosis. Adv Exp Med Biol 2021; 1301: 41-57.
11.
Zhou H, Zhou YL, Mao JA, et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol 2022; 55: 102413.
12.
Wang Y, Zhao Y, Ye T, Yang L, Shen Y, Li H. Ferroptosis signaling and regulators in atherosclerosis. Front Cell Dev Biol 2021; 9: 809457.
13.
Chen X, Xu S, Zhao C, Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun 2019; 516: 37-43.
14.
Ito J, Omiya S, Rusu MC, et al. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. Elife 2021; 10: e62174.
15.
Chen HY, Xiao ZZ, Ling X, Xu RN, Zhu P, Zheng SY. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 2021; 27: 14.
16.
Xiao Z, Kong B, Fang J, et al. Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 2021; 12: 9367-9376.
17.
Ying H, Shen Z, Wang J, Zhou B. Role of iron homeostasis in the heart : Heart failure, cardiomyopathy, and ischemia-reperfusion injury. Herz 2022; 47: 141-149.
18.
Kuno S, Fujita H, Tanaka YK, Ogra Y, Iwai K. Iron-induced NCOA4 condensation regulates ferritin fate and iron homeostasis. EMBO Rep 2022; 23: e54278.
19.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 2015; 59: 298-308.
20.
Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016; 12: 1425-1428.
21.
Xia R, Wang W, Gao B, et al. Moxibustion alleviates chronic heart failure by regulating mitochondrial dynamics and inhibiting autophagy. Exp Ther Med 2022; 23: 359.
22.
Wang W, Li QL, Ma Q, et al. Effects of moxibustion at bilateral Feishu (BL13) and Xinshu (BL15) combined with benazepril on myocardial cells apoptosis index and apoptosis-related proteins cytochrome c and apoptosis-inducing factor in rats with chronic heart failure. J Tradit Chin Med 2022; 42: 227-233.
23.
Li QL, Wang W, Ma Q, et al. Moxibustion Improves Chronic Heart Failure by Inhibiting Autophagy and Inflammation via Upregulation of mTOR Expression. Evid Based Complement Alternat Med 2021: 2021: 6635876.
24.
National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) Copyright© 2011, National Academy of Sciences.
25.
Zhang L, Gan ZK, Han LN, et al. Protective effect of heme oxygenase-1 on Wistar rats with heart failure through the inhibition of inflammation and amelioration of intestinal microcirculation. J Geriatr Cardiol 2015; 12: 353-365.
26.
Xie F, Wei K, Min S, et al. Anti-apoptotic effect of NGF on H9C2 cardiac myocytes in a hypoxia/reoxygenation injury model. Chinese Pharmacological Bulletin 2014; 30: 506-510.
27.
Bostan MM, Stătescu C, Anghel L, Șerban IL, Cojocaru E, Sascău R. Post-myocardial infarction ventricular remodeling biomarkers-the key link between pathophysiology and clinic. Biomolecules 2020; 10: 1587.
28.
Fratta Pasini AM, Stranieri C, Busti F, Di Leo EG, Girelli D, Cominacini L. New insights into the role of ferroptosis in cardiovascular diseases. Cells 2023; 12: 867.
29.
Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017; 113: 1708-1718.
30.
Kuwahara K. The natriuretic peptide system in heart failure: diagnostic and therapeutic implications. Pharmacol Ther 2021; 227: 107863.
31.
Zhang Y, Xin L, Xiang M, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother 2022; 145: 112423.
32.
Yu B, Wang X, Song Y, et al. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol Ther 2022; 238: 108186.
33.
Zhu F, Yuan C, Zhang X, Wang Z, Wang Q, Wang H. A-kinase anchoring protein 5-ancored calcineurin regulates the remodeling of H9c2 cardiomyocytes exposed to hypoxia and reoxygenation. Biomed Pharmacother 2022; 155: 113689.
34.
Peng M, Liu Y, Zhang XQ, Xu YW, Zhao YT, Yang HB. CTRP5-overexpression attenuated ischemia-reperfusion associated heart injuries and improved infarction induced heart failure. Front Pharmacol 2020; 11: 603322.
35.
Zhu HJ, Han ZY, He SF, et al. Specific MicroRNAs comparisons in hypoxia and morphine preconditioning against hypoxia-reoxgenation injury with and without heart failure. Life Sci 2017; 170: 82-92.
36.
Zhang P, Liao J, Wang X, Feng Z. High glucose promotes apoptosis and autophagy of MC3T3-E1 osteoblasts. Arch Med Sci 2020; 19: 138-150.
37.
Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 2023; 20: 7-23.
38.
Kanamori H, Yoshida A, Naruse G, et al. Impact of autophagy on prognosis of patients with dilated cardiomyopathy. J Am Coll Cardiol 2022; 79: 789-801.
39.
Yue H, Zhan Y, Zhang Z, Liang W, Wu Z. The emerging role of ferroptosis in myocardial fibrosis of atrial fibrillation. Arch Med Sci 2023; 19: 507-512.
40.
Ghafourian K, Shapiro JS, Goodman L, Ardehali H. Iron and heart failure: diagnosis, therapies, and future directions. JACC Basic Transl Sci 2020; 5: 300-313.
41.
Yang X, Kawasaki NK, Min J, Matsui T, Wang F. Ferroptosis in heart failure. J Mol Cell Cardiol 2022; 173: 141-153.
42.
Zhang B, Pan C, Feng C, et al. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep 2022; 27: 45-52.
43.
Ma S, Dielschneider RF, Henson ES, et al. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One 2017; 12: e0182921.
44.
Zhou Y, Shen Y, Chen C, et al. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Cancer Biol Med 2019; 16: 630-646.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.